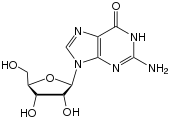
Guanine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Guanine | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 5 H 5 N 5 O | |||||||||||||||||||||
| Brief description |
white to yellowish white solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 151.13 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
> 365 ° C (decomposition) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Guanine (G, Gua) is one of the four nucleobases in DNA and RNA , along with adenine , cytosine and thymine ( uracil in RNA). It is a heterocyclic organic compound with a purine backbone and two substituents ( amino group in position 2 and oxygen atom in position 6). The nucleoside of guanine is the deoxyguanosine in DNA and the guanosine in RNA. In the Watson-Crick base pairing , it forms three hydrogen bonds with cytosine.
Biosynthesis and metabolism
The organism of most living things is able to synthesize guanine itself; Since this metabolic pathway is very energy-intensive, the body can also reuse the purine derivative guanine via the salvage pathway , cut it from nucleic acid molecules to be broken down and, if necessary, bind it again to ribose.
The breakdown of guanine takes place to xanthine and further to uric acid .
In reptiles and birds represents guanine next uric an important excretion form of nitrogen (mainly of the protein needs to be is the elimination product is pasty and contains little water, so not carried its mass, what the flying supported - and Nukleotidabbau).. This is more energy-efficient than using the chemical energy contained in guanine. For the same reason, bats also secrete guanine. Excreted guanine (and uric acid) forms guano after weathering , especially on calcareous soils.
presentation
A representation takes place via the grape synthesis by heating 2,4,5-triamine-1,6-dihydro-6-oxopyrimidine (as sulfate ) with formic acid for several hours.
properties
Guanine is a white to yellowish white solid that melts at over 365 ° C with decomposition. It is insoluble in water, soluble in dilute acids and alkalis, sparingly soluble in ethanol and diethyl ether .
History and biological meaning
In 1871 Friedrich Miescher reported on a substance containing phosphorus that he was able to isolate from the cell nuclei of erythrocytes. He called her nucleus. In 1883 Albrecht Kossel , who later won the Nobel Prize, was able to prove that guanine is the fission product of a nuclein obtained from goose blood. Guanine has been known since 1844 as a nitrogen-rich base that accumulates in the excrement of mammals and birds. The first findings on the occurrence of guanine in the nucleus had been around since 1874. They went back to the Swiss chemist Jules Piccard , who, at the request of Friedrich Miescher, had examined the “nucleic acid of salmon sperm”. Kossel demonstrated for the first time in 1891 that guanine is a cleavage product of nucleic acid obtained from yeast .
Guanine can be part of DNA, RNA or various nucleosides and nucleotides .
Nucleosides
Via the N 9 atom of the five-membered ring, guanine can be bound N -glycosidically to the C 1 atom of the ribose ; one then speaks of a nucleoside , guanosine . When it binds to deoxyribose , the nucleoside deoxyguanosine is formed .
Nucleotides
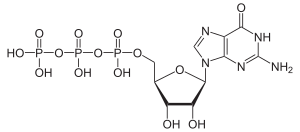
On the phosphorylation of guanosine at the C 5 atom of the ribose leads to important nucleotides guanosine monophosphate (GMP), guanosine diphosphate (GDP) and guanosine triphosphate (GTP), or by analogy to the deoxyguanosine to deoxyguanosine monophosphate (dGMP) Desoxyguanosindiphosphat (DGDP) and Deoxyguanosine triphosphate (dGTP).
Part of DNA and RNA
In the DNA double helix , guanine forms three hydrogen bonds with the corresponding cytosine base of the complementary strand via the oxo group, the N 1 atom and the amino group .
Pathophysiology
If the salvage pathway is disturbed, especially if the enzyme hypoxanthine-guanine-phosphoribosyl-transferase ( HGPRT ) is defective , the clinical picture of Lesch-Nyhan syndrome occurs .
If the amount of metabolized guanine is too high, the resulting non-physiologically high amount of uric acid ( hyperuricemia ) can crystallize in the kidneys, the urinary tract or in bradytrophic tissues (especially joint capsules) and lead to urinary stones or gout .
Related links

|

|
|

|

|
|
| 1-methylguanine | 7-methylguanine | N 2 methylguanine | N 2 , N 2 -dimethylguanine | 6- O -methylguanine | Isoguanine |
Individual evidence
- ↑ Entry on CI 75170 in the CosIng database of the EU Commission, accessed on March 28, 2020.
- ↑ a b c d Entry on guanine. In: Römpp Online . Georg Thieme Verlag, accessed on November 11, 2014.
- ↑ Guanine data sheet (PDF) from Carl Roth , accessed on December 14, 2010.
- ↑ a b Entry on guanine in the GESTIS substance database of the IFA , accessed on February 13, 2017(JavaScript required) .
- ↑ Miescher, F .: About the chemical composition of the pus cells. In: Hoppe-Seyler's medical-chemical investigations , No. 4, 1871, pp. 441-460.
- ↑ Kossel, A .: On the chemistry of the cell nucleus. In: Zeitschrift für Physiologische Chemie , Volume 7, 1882-83, p. 7.
- ↑ Piccard, J .: About protamine, guanine and sarkine, as components of salmon sperm. In: Reports of the German Chemical Society , 1874, p. 1714.
- ↑ Kossel, A .: About the chemical composition of the cell. Lecture in: Archives for Anatomy and Physiology. Physiological Department , 1891, p. 178.
Web links
- Entry for guanine in the Human Metabolome Database (HMDB) , accessed November 18, 2013.