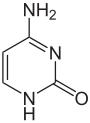
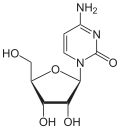
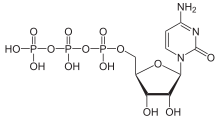
Cytosine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
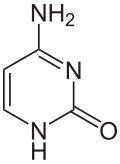
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cytosine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 5 N 3 O | ||||||||||||||||||
| Brief description |
colorless platelets |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 111.10 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| solubility |
moderately in boiling water and ethanol , insoluble in diethyl ether |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−221.3 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Cytosine (C, Cyt) is one of the four nucleobases in DNA and RNA , along with adenine , guanine and thymine ( uracil in RNA). It is a heterocyclic organic compound with a pyrimidine backbone and two substituents ( amino group in position 4 and oxygen atom in position 2). The nucleoside of cytosine is the deoxycytidine in DNA and the cytidine in RNA. In the Watson-Crick base pairing , it forms three hydrogen bonds with guanine.
History, extraction and representation
The cytosine was first obtained from the thymus gland of calves in 1894 by the later Nobel Prize winner Albrecht Kossel and his assistant Albert Neumann .
In 1903 the chemical structure was clarified by Albrecht Kossel in collaboration with Hermann Steudel and the first successful synthesis was carried out.
properties
Physical Properties
Cytosine forms colorless platelets with a melting point of over 300 ° C. It dissolves moderately in boiling water and ethanol ; it is insoluble in diethyl ether .
Cytosine is tautomeric , with the 1 H form predominating.
Chemical properties
Due to its chemical instability cytosine can uracil deamination .
Biological importance
Cytosine can be part of DNA, RNA or various nucleosides and nucleotides .
Nucleosides
Via the N 1 atom of the ring, cytosine can be bound N -glycosidically to the C 1 atom of the ribose ; one then speaks of a nucleoside , the cytidine . The nucleoside deoxycytidine is formed when it binds to deoxyribose . If, however, cytosine is C-glycosidically bound to the C 1 atom of the ribose via the C 5 atom of the ring , the synthetic pseudocytidine is formed . In contrast to most nucleosides, cytarabine contains arabinose instead of ribose .
Nucleotides
The phosphorylation of cytidine at the C 5 atom of ribose leads to the important nucleotides cytidine monophosphate (CMP), cytidine diphosphate (CDP) and cytidine triphosphate (CTP), or similarly for deoxycytidine to deoxycytidine monophosphate (dCMP), deoxycytiphosphate ( dCMP), deoxycytosphidiphosphate (dCTP).
As cytidine triphosphate (CTP) it serves as a cofactor for various enzymes and can give its phosphate group to ADP to build up ATP .
Part of DNA and RNA
In the DNA double helix , cytosine forms three hydrogen bonds with the corresponding guanine base of the complementary strand via the oxo group, the N 3 atom and the amino group .
It is converted into the methylated form 5-methylcytosine by cytosine-specific methyltransferases .
Related links
Individual evidence
- ↑ Entry on CYTOSINE in the CosIng database of the EU Commission, accessed on March 31, 2020.
- ↑ a b c Entry on cytosine. In: Römpp Online . Georg Thieme Verlag, accessed on November 11, 2014.
- ↑ A. Abdelaziz, DH Zaitsau, TA Mukhametzyanov, BN Solomonov, P. Cebe, SP Verevkin, C. Schick: Melting temperature and heat of fusion of cytosine revealed from fast scanning calorimetry ; in: Thermochim. Acta , 2017, 657, pp. 47-55 ( doi : 10.1016 / j.tca.2017.09.013 ).
- ↑ a b Cytosine data sheet from Sigma-Aldrich , accessed on November 23, 2013 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-25.
- ↑ Representation and cleavage products of nucleic acid (adenylic acid). Lecture in: Reports of the German Chemical Society , Volume 27, 1894, page 2215.
- ↑ A. Kossel , H. Steudel: Further investigations on cytosine ; in: Hoppe-Seyler's magazine for physiological chemistry , 1903 , 38 (1-2), pp. 49-59 ( doi : 10.1515 / bchm2.1903.38.1-2.49 ).
- ↑ Further research on cytosine. In: Hoppe-Seyler's magazine for physiological chemistry , Volume 38, 1903, page 49.
- ↑ A. Kossel , H. Steudel: About a basic component of animal cells ; in: Hoppe-Seyler's magazine for physiological chemistry , 1903 , 37 (2), pp. 177-180 ( doi : 10.1515 / bchm2.1903.37.2.177 ).
- ↑ HL Wheeler, TB Johnson: Synthesis of aminooxy-pyrimidines having the composition of cytosine, 2-amino-6-oxypyrimidine and 2-oxy-6-amino-pyrimidine ; in: Am. Chem. J. , 1903 , 29 , pp. 492-504.
- ↑ M. Dreyfus, O. Bensaude, G. Dodin, JE Dubois: Tautomerism in Cytosine and 3-Methylcytosine. A Thermodynamic and Kinetic Study ; in: J. Am. Chem. Soc. , 1976 , 98 (20), pp. 6338-6349 ( doi : 10.1021 / ja00436a045 ; PMID 965648 ).
- ^ GA Wagner: Introduction to Archaeometry , 1st edition, Springer Verlag, Berlin 2007, ISBN 3-540-71936-9 , p. 282.
- ^ JR Siewert, M. Rothmund, V. Schumpelick: Praxis der Viszeralchirurgie: Onkologische Chirurgie , 3rd edition, Springer Verlag, Berlin 2010, ISBN 3-642-03807-7 , p. 71.
Web links
- Entry on cytosines in the Human Metabolome Database (HMDB) , accessed November 18, 2013.