Arabinose
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| Fischer projection , open-chain representation | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Arabinose | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 10 O 5 | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 150.13 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.6 g cm −3 |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| solubility |
59.4 g / 100 g water at 10 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
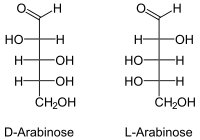
Arabinose is a naturally occurring simple sugar ( monosaccharide ) that consists of five carbon atoms ( pentose ). Arabinose is sometimes referred to as pectinose , gum sugar or aloin sugar .
Constitution and stereochemistry
The open-chain arabinose is diastereomeric to the three other aldopentoses ribose , xylose and lyxose . As a chiral compound, it consists of the two enantiomers D - (-) - arabinose and L - (+) - arabinose.
The two cyclic constitutional isomers arabinofuranose and arabinopyranose are obtained through intramolecular hemiacetal formation . Since a further center of asymmetry arises during hemiacetal formation , two diastereomers each result, α- and β-arabinofuranose, and α- and β-arabinopyranose. The α- and β-furanose or α- and β-pyranose pairs are also referred to as anomers .
| D -Arabinose - spellings | ||
|---|---|---|
| Wedge formula | Haworth notation | |

|
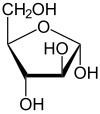 α- D -arabinofuranose |
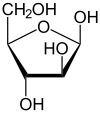 β- D -arabinofuranose |
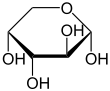 α- D -arabinopyranose |
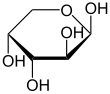 β- D -arabinopyranose |
|
Each ring shape and each stereoisomer has its own CAS number:
| open- chain |
Furanosis | Pyranosis | |||
|---|---|---|---|---|---|
| α | β | α | β | ||
| D -arabinosis | 10323-20-3 | 37388-49-1 | 25545-03-3 | 608-45-7 | 6748-95-4 |
| L -arabinosis | 5328-37-0 | 38029-69-5 | 20074-49-1 | 7296-55-1 | 7296-56-2 |
properties
In aqueous solution, the anomeric arabinofuranoses and arabinopyranoses are in equilibrium with one another via the unstable open-chain aldehyde form. The equilibrium is dominated to 61% by α-arabinopyranose, followed by β-arabinopyranose with 35%. The two arabinofuranoses only play a minor role with 2% each. The establishment of equilibrium is called mutarotation .
Arabinose can not be fermented by ordinary yeast . In 2005, researchers at the Goethe University in Frankfurt am Main succeeded in modifying yeast cultures so that they can also ferment arabinose and xylose to produce ethanol .
Occurrence
The L -form occurs more frequently in food, mostly as a component in polysaccharide chains in the plant kingdom. Like other sugars, it has a sweet taste. The D form was as part of a polysaccharide in tuberculosis - bacilli detected.
Dismantling
The breakdown of L -arabinose in E. coli is controlled by the ara operon . Is L -arabinose present in the cell, which is transcription of a promoter activated and the synthesized mRNA derived from the gene araA apparent, may, after translation in the L -arabinose isomerase to be converted. The L -arabinose isomerase converts L -arabinose into L - ribulose .
See also
Web links
Individual evidence
- ↑ Data sheet arabinose (PDF) from Carl Roth , accessed on March 23, 2007.
- ↑ Data sheet D-arabinose at Sciencelab ( Memento from March 4, 2016 in the Internet Archive ).
- ↑ a b c Entry on arabinose. In: Römpp Online . Georg Thieme Verlag, accessed on February 20, 2019.
- ↑ a b Data sheet D - (-) - Arabinose from AlfaAesar, accessed on February 20, 2019 ( PDF )(JavaScript required) .
- ↑ Eberhard Breitmeier, Günther Jung: Organic chemistry . Basics, substance classes, reactions, concepts, molecular structure. 5th edition. Georg Thieme Verlag, Stuttgart 2005, ISBN 3-13-541505-8 , p. 851 ( limited preview in Google Book search).
- ↑ Scientists develop a new type of yeast through "controlled evolution" Genetic engineering yeast turns plant waste into biofuel. scinexx | The knowledge magazine, accessed on January 24, 2020 .
- ↑ L-arabinose | GoldBio. Retrieved March 5, 2020 .
- ↑ James W. Patrick, Nancy Lee: Purification and Properties of an I-Arabinose Isomerase from Escherichia coli . In: Journal of Biological Chemistry . tape 243 , no. 16 , 25 August 1968, ISSN 0021-9258 , p. 4312-4318 , PMID 4878429 ( jbc.org [accessed March 5, 2020]).
