Monosaccharides
Monosaccharides ( old Gr . Μόνος mónos 'alone', τό σάκχαρ tó sákchar 'sugar' and -ειδής -eidés 'like, shaped' also called simple sugars ) are a group of substances made up of organic chemical compounds. They are the products of the partial oxidation of polyhydric alcohols. All monosaccharides have a chain of at least three carbon atoms as a basic structure and have a carbonyl group and at least one hydroxyl group . They are the building blocks of all carbohydrates and can combine to form disaccharides ( double sugars ), oligosaccharides ( multiple sugars ) or polysaccharides ( multiple sugars ).
The monosaccharides glucose , fructose and galactose are the most important sugars in the metabolism . They are energy carriers and also serve as cell building blocks.
construction
Every simple sugar consists of a chain of carbon atoms. The chain can be open (non- cyclic) or closed in a ring (cyclic) .
According to the number of carbon atoms one speaks of dioses (2), trioses (3), tetroses (4), pentoses (5), hexoses (6), heptoses (7) etc. The smallest simple sugars, the triose , have three carbon atoms . In principle, the length of the carbon chain is unlimited, but so far only simple sugars with a maximum of nine carbon atoms have been observed in nature, with hexoses and pentoses being the most common.
Furthermore, one of the carbon atoms of the non-cyclic (open-chain) form has a double-bonded oxygen atom, i.e. a carbonyl group. If the carbonyl group is at the end of the carbon chain, the group is called the aldehyde group and the sugar is called the aldose , with a carbonyl group within the chain from a keto group and with the sugar from ketoses .
Both nomenclatures can be used together, so that a simple sugar with six carbon atoms and one aldehyde group is called an aldohexose.
For every open-chain monosaccharide of sufficient length there are cyclic forms, with the ring closure from the carbonyl group to a hydroxyl group (OH group). The ring thus consists of carbon atoms and one oxygen atom. A distinction is made between furanoses (five rings) and pyranoses (six rings). Aldoses derived cyclic monosaccharides are hemiacetals derived from ketoses hemiketals .
In the simplest representatives of the monosaccharides, the remaining carbon atoms without a carbonyl group each have a hydroxyl group (OH group) and otherwise have hydrogen atoms. The general empirical formula applies to these compounds : C n H 2n O n .
However, derivatives of these simple compounds also belong to the monosaccharides, such as amino sugars (e.g. glucosamine ) and deoxy sugars (e.g. deoxyribose ). They do not correspond to this general molecular formula.
Spatial structure
In addition to the position of the carbonyl group (oxo group) in the carbon chain , the spatial arrangement of the OH groups also plays an important role. In the case of an aldohexose, for example, four of the carbon atoms can be distinguished depending on the OH group attached to the right or left. In total there are 16 (2 4 = 16) different stereoisomers of an aldohexose, which differ in terms of metabolism and optical activity . Furthermore, the number of theoretically possible stereoisomers is increased fivefold, since in addition to the open-chain form, the furanose or pyranose form with an α or β configuration can arise through the intramolecular formation of a cyclic hemiacetal .
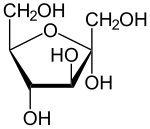
The stereochemical representation can be done in three equal ways. The oldest representation is the Fischer projection , in which all CC bonds are written in an imaginary (thermodynamically most unfavorable) eclipsed position vertically one above the other and rolled out on the plane of the paper. The substituents , here hydrogen atoms and hydroxyl groups, are listed on the right or left depending on the configuration and are above the plane of the paper, so that a clear configuration results. The hydroxyl group of the chiral C atom furthest from the anomeric C atom forms the D configuration in the right position and the L configuration in the left position (see the example of D and L ribose above).
The cyclic hemiacetal is confusing in the representation ( 1 ), which is also called Tollens' ring formula, and excessively long bonds are required. Therefore, further representations were developed. The Haworth representation ( 2 ) corresponds to a Fischer projection “laid on its side” and rolled up. All ring atoms are on one level, the spatial impression can be enhanced by perspective bonds. The binding of a substituent upwards is intended to indicate that it is above the plane of the ring. The groups pointing to the left (or right) in the Fischer projection point up (or down) on Haworth-Ring.
The conformational formula ( 3 ) is even more realistic , as the angled arrangement of the carbon chain can be seen here. The stereochemical representation ( 4 ) is also common.
Important monosaccharides
Family tree of aldoses and ketoses
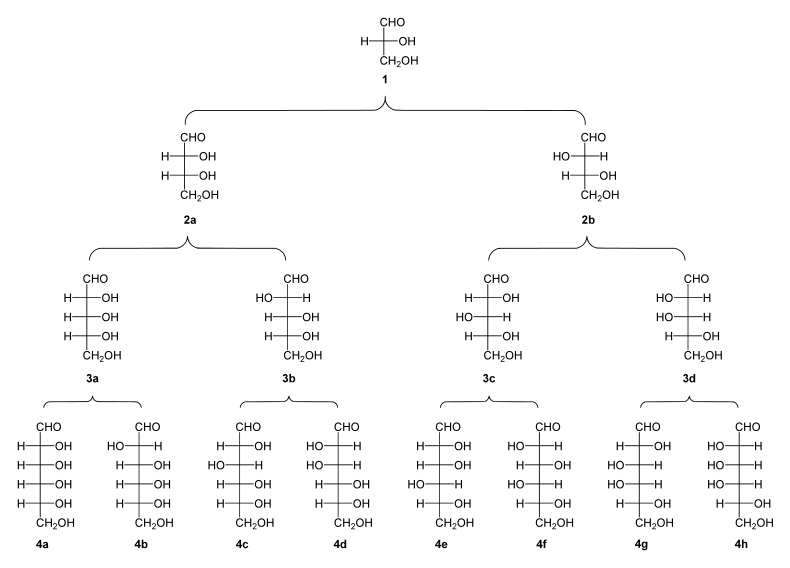
“Pedigree” of the D alcases. By adding CH – OH groups, the basic structure is extended so that further sugars can be derived (from trioses with three C atoms to hexoses with six C atoms). The direction of rotation of polarized light is indicated by (+) or (-).
( 1 ) D - (+) - glyceraldehyde ; ( 2a ) D - (-) - erythrosis ; ( 2b ) D - (-) - threose ; ( 3a ) D - (-) - ribose ; ( 3b ) D - (-) - arabinose ; ( 3c ) D - (+) - xylose ; ( 3d ) D - (-) - lyxose ; ( 4a ) D - (+) - allose ; ( 4b ) D - (+) - alto rose ; ( 4c ) D - (+) - glucose ; ( 4d ) D - (+) - mannose ; ( 4e ) D - (-) - gulose ; ( 4f ) D - (-) - idose ; ( 4g ) D - (+) - galactose ; ( 4h ) D - (+) - Talose |

"Pedigree" of the D -Ketoses. By adding CH – OH groups, the basic structure is extended so that further sugars can be derived (from trioses with three C atoms to hexoses with six C atoms).
( 1 ) dihydroxyacetone ; ( 2 ) D - erythrulose ; ( 3a ) D - ribulose ; ( 3b ) D - xylulose ; ( 4a ) D - psicose ; ( 4b ) D - fructose ; ( 4c ) D - sorbose ; ( 4d ) D - Tagatose |
Trios
-
Aldotriosis
- D - and L - glyceraldehyde ( metabolic products )
-
(Ketotriosis)
- ( Dihydroxyacetone ) (Since the dihydroxyacetone does not have a stereocenter, it is actually not considered a monosaccharide. However, it is involved and important in the carbohydrate metabolism.)
Tetroses
-
Aldotetrose
- D - erythrosis (metabolic product)
- D - threose (metabolic product)
-
Ketotetrose
- D - erythrulose (metabolic product )
Pentoses
-
Aldopentosis
- D - ribose (occurs among other things in the RNA )
- D - and L - arabinose (found in vegetable oligosaccharides)
- D - xylose (also wood sugar, main component of hemicelluloses )
- D - Lyxosis (does not occur in nature)
- D - deoxyribose (occurs among other things in the DNA )
-
Ketopentosis
- D - ribulose (metabolic product)
- D - and L - xylulose (the former is part of the pentose phosphate pathway , the latter of the glucuronate metabolism)
Hexoses
-
Aldohexoses
- D - Allose (very rare in nature)
- D - Altrose (not found in nature, L-Altrose was found in a bacterium)
- D - glucose (also grape sugar, most common monosaccharide)
- D - mannose (common monosaccharide)
- D - Gulose (rare in nature, mostly in L-shape)
- D - Idose (does not occur in nature, but the corresponding uronic acid, iduronic acid , occurs mainly in glycosaminoglycans )
- D - galactose (also mucus sugar, common monosaccharide)
- D - Talose (very rare, part of the antibiotic hygromycins formed by streptomycetes )
-
other physiologically important hexoses
- D - glucuronic acid (6-carboxy- D -glucose, often, is usually present as glucuronate or esterified )
- D - galacturonic acid (6-carboxy- D -galactose, is usually present as uronate or esterified)
- N -acetyl-D-glucosamine (also N -acetylchitosamine, monomer of chitin , occurs widely)
- D - glucosamine (also chitosamine, monomer of chitosan )
- N -acetyl-D-galactosamine (also N -acetylchondrosamine, is widespread)
- D - and especially L - fucose (6-deoxy- D - and - L -galactose, the latter is widely distributed)
- L - rhamnose (6-deoxy- L -mannose, occurs in vegetable oligosaccharides)
- D - Chinovose (6-deoxy- D -glucose, occurs e.g. in vegetable oligosaccharides)
-
Ketohexoses
- D - fructose , (also fruit sugar, common monosaccharide)
- Rare hexoses
Higher monosaccharides
- D - Sedoheptulose (a C 7 keto sugar, is involved in the pentose phosphate pathway as 7-phosphate )
- 3-deoxy-D- manno- oct-2-ulosonic acid (also 2-keto-3-deoxyoctonic acid, KDO, a C 8 sugar, an important component of lipopolysaccharides on the cell surface of certain bacteria)
- D - sialic acid (also N- acetyl neuraminic acid , a C 9 keto sugar, plays a role in cell-cell recognition in glycoconjugates )
photosynthesis
The starting point of most simple sugars in living things is oxygenic photosynthesis . During this process, using solar energy, sugar is built up from CO 2 ( carbon dioxide ) and the hydrogen atoms contained in H 2 O ( water ) . During the splitting of water required for this, oxygen is released as a waste product .
Simple sugars in foods
Simple sugars are found as glucose (grape sugar) and fructose (fruit sugar) in foods such as fruit, honey and sweets. The galactose , called mucus sugar in milk, is a simple sugar. In contrast, cane , milk or malt sugars are double sugars; Starch and glycogen are polysaccharides. All higher sugars must first be broken down into water-soluble di- or monosaccharides in order to be absorbed into the blood or transported into the liver via transport proteins - such as the glucose transporter - or simple diffusion.
Oral consumption of the monosaccharides glucose and galactose leads to a rapid rise in blood sugar levels ; all other simple sugars are mainly metabolized in the liver and have no direct effect on the glucose level in the blood. Since the blood sugar level has to fluctuate within a narrow range, it is necessary for the organism to counteract the rapid rise through rapid further processing. The insulin level is rising and the blood sugar in the liver glycogen - a polysaccharide of glucose units - converted. This creates a fast energy store in the liver, as the glycogen can be quickly broken down into glucose again if required. Excess glucose, which cannot be stored as glycogen, is converted in adipose tissue and the liver into triacylglycerols ( fats ), which serve as an energy store in the liver, skeletal muscles and fat cells .
Nutritionists recommend consuming a maximum of 10% of the total amount of energy through simple and double sugars. Polysaccharides like v. a. Starches are considered to be better suited to meet carbohydrate requirements, as they first have to be converted into simple sugars in the gastrointestinal tract, which leads to a significantly slower absorption of the carbohydrates.
literature
- Gerhard Richter: Metabolic Physiology of Plants. Physiology and biochemistry of primary and secondary metabolism . 6th, completely revised edition. Georg Thieme, Stuttgart et al. 1998, ISBN 3-13-442006-6 , p. 213–223 ( limited preview in Google Book search).
Web links
Individual evidence
- ↑ Espacenet - Bibliographic data. Retrieved November 18, 2018 .
- ^ Hans-Dieter Belitz , Werner Grosch, Peter Schieberle : Textbook of food chemistry. 6th, completely revised edition. Springer, Berlin et al. 2008, ISBN 978-3-540-73201-3 , p. 895.
- ↑ Georg Löffler, Petro E. Petrides, Peter C. Heinrich (Eds.): Biochemistry and Pathobiochemistry. 8th, completely revised edition. Springer Medicine, Heidelberg 2007, ISBN 978-3-540-32680-9 .
- ^ Hermann Hager (founder), Hubert Schneemann, Gisela Wurm: Hager's handbook of pharmaceutical practice. Follow-up work, follow-up volume 1: Goods and services. 5th, completely revised edition. Springer, Berlin et al. 1995, ISBN 3-540-58958-9 , p. 18.
- ↑ Report of the Food and Agriculture Organization of the United Nations: Diet, Nutrition and the Prevention of Chronic Diseases (Table 6)

