Blood sugar
Under blood sugar is generally understood as the amount of glucose in the blood . Glucose is an important source of energy for the body. The brain , the red blood cells and the kidney medulla are dependent on glucose for energy production, all other body cells obtain the energy primarily in the fat metabolism . Glucose is able to cross the blood-brain barrier and thus supplies the brain.
In medicine, the blood sugar level ( blood sugar level , glucose level ) is an important measured value. If it is permanently elevated, diabetes mellitus may be present.
A low blood sugar may reduce the brain function that cause seizures, increased adrenaline and shaky hands and sweats. In pronounced form, the hypoglycaemia leads to shock . It is typically found in the very rare insulinoma , but in some cases also as an early symptom of type 2 diabetes, rarely even without further illnesses after a meal with rapidly absorbable carbohydrates . It is a common complication of some drugs in the treatment of diabetes mellitus.
Blood glucose testing
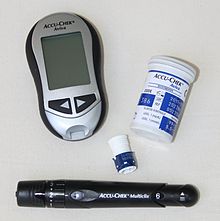
Blood sugar is measured from a blood sample , usually from capillary blood . From the point of view of measurement accuracy, a distinction must be made between measurements by the patient himself with the help of blood glucose meters and higher-quality measurements in the laboratory.
As a unit in most countries, the SI-compliant (will International System of Units ) Unit mmol / L ( millimoles per liter). In the western part of Germany (and Berlin), as in the USA, Poland, France, Italy, Japan or Austria, the older (but also SI-compliant) unit mg / dl (milligrams per deciliter, synonymous with this is not) SI-compliant unit mg% ) is used.
For a while, measuring devices were in use that could display the result either in mg / dl or in mmol / l. According to the Federal Institute for Drugs and Medical Devices , this led to mix-ups of the underlying unit of measurement in several cases, which resulted in incorrect dosing of the insulin . Therefore, convertible devices have been taken off the market since the fourth quarter of 2006. There was a similar problem in Switzerland . Conversion:
The blood sugar level can be determined very quickly and largely reliably with small blood sugar measuring devices, which are optionally available with units of measurement programmed by the manufacturer either in mmol / l or mg / dl, also on prescription.
Normal values
In humans, the normal values are:
- fasting: 70–99 mg / dl, corresponding to 3.9–5.5 mmol / l
- after a high-carbohydrate meal:
- maximum up to 160 mg / dl, corresponding to 8.9 mmol / l
- below 140 mg / dl after 2 hours, corresponding to 7.8 mmol / l
However, the values differ depending on the literature source and the test material (venous plasma, venous whole blood or capillary whole blood - see tables). Fasting blood sugar (NBZ) values> 5.5 mmol / l or> 99 mg / dl (according to other sources> 6.1 mmol / l or 110 mg / dl) indicate impaired glucose tolerance, fasting values> 125 mg / dl dl or> 6.9 mmol / l to diabetes mellitus . As a complication, significantly increased values in the context of wound treatment lead to prolonged or impaired wound healing. If necessary, when diabetes mellitus is diagnosed, treatment with insulin to support wound healing is necessary .
| classification | Fasting blood sugar (NBZ, venous) |
Blood sugar 2 hours after eating (or oGTT ) (venous) |
|---|---|---|
| normal | <110 mg / dl <6.1 mmol / l |
<140 mg / dl <7.8 mmol / l |
| Abnormal Fasting Glucose (IFG) |
110-125 mg / dl 6.1-6.9 mmol / l |
<140 mg / dl <7.8 mmol / l |
| Impaired glucose tolerance (IGT) |
<126 mg / dl <7.0 mmol / l |
140-200 mg / dl 7.8-11.1 mmol / l |
| Diabetes mellitus | ≥ 126 mg / dl ≥ 7.0 mmol / l |
≥ 200 mg / dl ≥ 11.1 mmol / l |
Abbreviations in the table above
- IFG = impaired fasting glucose (literally: disturbed fasting glucose)
- IGT = impaired glucose tolerance (literally: impaired glucose tolerance)
| Blood sugar control | Metabolism healthy |
|---|---|
| Blood sugar sober | 65-100 mg / dl 3.6-5.6 mmol / l |
| Blood sugar after eating |
80-126 mg / dl 4.5-7.0 mmol / l |
| Blood sugar at night | 65-100 mg / dl 3.6-5.6 mmol / l |
|
HbA 1c value (standardized value according to DCC trials) |
<6.05 |
| Measurement | Normal values | Suspicion / prediabetes |
Diabetes mellitus |
|
|---|---|---|---|---|
| sober | <100 mg / dl <5.6 mmol / l |
100-126 mg / dl 5.6-7.0 mmol / l |
> 126 mg / dl > 7.0 mmol / l |
|
| 2 hours after eating or in the oGTT |
capillary | <140 mg / dl <7.8 mmol / l |
140-200 mg / dl 7.8-11.1 mmol / l |
> 200 mg / dl > 11.1 mmol / l |
| venous | <120 mg / dl <7.0 mmol / l |
120-180 mg / dl 7.0-10.0 mmol / l |
> 180 mg / dl > 10.0 mmol / l |
|
| HbA 1c | <6.5% | 6.5-7.5% | > 7.5% | |
A blood sugar level that is too high is called hyperglycemia , and hypoglycemia that is too low . A special form of hemoglobin , HbA1c , is able to reproduce the blood sugar course over a maximum of three months and is therefore also called "blood sugar memory". Hemoglobin is the red blood pigment in the erythrocytes that carries oxygen. HbA1c is hemoglobin that has been non-enzymatically glycated due to an excessively high blood sugar concentration . The HbA1c provides information about the last three months, since the lifespan of the erythrocytes is 120 days.
Measurement methods
There are essentially three measurement methods that have become established for self- monitoring blood glucose meters .
Optical measurement
With the optical measurement, the blood in the test strip is drawn in via a capillary to an externally visible test field. Various chemical substances are stored there that react with the blood and change the color of the test field. This color change is recorded by the measuring device and determined from the duration and strength of the change in the blood sugar value.
Amperometric measurement
With amperometric measurement, the blood in the test strip is sucked into a test field via a capillary . In the test field, the blood is in contact with glucose oxidase and various electrodes . The measuring device applies a defined electrical voltage (approx. 300–600 mV) to these electrodes and measures the current that flows over the electrodes over time . The device determines the blood sugar value from the measured current. The current is proportional to the glucose concentration of the liquid in the containment (sensor area of the capillary).
Non-invasive measurement
With injury-free, so-called non-invasive methods, the blood sugar level can be displayed, tracked or recorded over time without having to draw blood. With these and similar measuring methods, permanent recording or display of the time course (monitoring) of the blood sugar level is basically possible.
- With a broadband laser in the mid-infrared range (MIR), the blood sugar level can be measured through the skin without injury using "multi-wavelength densitometry".
- An optical spectral analysis of the fundus of the eye, which is well supplied with blood, can provide very precise values. An implanted passive microsensor in the eye can also increase the quality of the measurements.
- With a permanently implanted microspectrometer without moving components, the spectroscopic measurement of blood sugar in the near infrared range (NIR) can be carried out ( IR spectroscopy ). This sensor transmits its measured values to a display device with a passive transponder .
These and other non-invasive methods are still being researched or are in clinical approval (particularly in the USA). A glucose-sensitive nanosensor was newly developed at the American Northeastern University in Boston, the nanoparticles of which are injected like a tattoo and which fluoresce when blood sugar levels are high. A method is being developed at Brown University that uses plasmon interferometry to measure the glucose content of saliva .
A market launch of non-invasive blood glucose measurement using spectroscopic measurement methods in the near-infrared (NIR) range with extracorporeal measuring devices has so far failed because the devices cannot detect tissue sugar, i.e. H. Determine glucose per volume of the body tissue irradiated and not the blood sugar per blood volume, because the measuring beam has to penetrate the body tissue for the measurement.
Determination of glucose in urine
It is also possible to measure the urinary glucose value. However, glucose can only be detected in the urine if the glucose concentration is greatly increased and has exceeded a certain value. This value depends on the so-called kidney threshold of the respective test person . However, this kidney threshold is very unreliable and easily disruptive. During pregnancy , for example, the kidney threshold can drop to below 120 mg / dl, and in healthy people it can be over 200 mg / dl. Even mild kidney disease can change the kidney threshold. Due to the relatively high reliability and good availability of the blood sugar measuring devices at the same high prices for the measuring strips, the determination of the urine sugar can be regarded as outdated.
regulation
The blood sugar level is regulated by the interplay of two peptide hormones in the pancreas . This gland contains blood sugar sensor systems in its α and β cells , which respond as follows:
- when the sugar level in the blood drops ("hunger signal"), glucagon is secreted . This hormone activates glycogen phosphorylase (PYG) in the liver , which initiates the breakdown of glycogen to glucose ( catabolic branch ) (top picture)
- When the blood sugar level rises, insulin is secreted, which initiates a series of glucose-consuming reactions, especially in the liver ( anabolic branch ). Of central importance here is the indirect activation of glycogen synthase (GYS), which uses the excess glucose to build up the energy store glycogen ("animal starch") (lower picture)
In addition, adrenaline activates glycogen phosphorylase in skeletal muscle cells. Elevated adenosine monophosphate levels in the liver and muscles also activate the enzyme, as does the release of calcium from the sarcoplasmic reticulum with subsequent binding to calmodulin .
Glycogen breakdown and build-up are strictly counter-regulated via the phosphorylation of the key enzymes glycogen phosphorylase (PYG) and glycogen synthase (GYS), so they never run simultaneously. In energy deficit situations, both enzymes are phosphorylated by kinases; this process stimulates phosphorylase but inhibits synthase. If there is an excess of glucose, the situation is reversed by the action of phosphatases : Loss of phosphate residues inactivates PYG, but activates GYS.
Both the glucagon and the insulin signal are amplified via signal cascades. Protein kinases are at the center of both signaling pathways: each kinase phosphorylates several molecules of a downstream kinase.
- In the case of glucagon or adrenaline, a G protein- dependent receptor ( GPCR , seven transmembrane helix type) is activated. Adenylate cyclase , an enzyme that produces the second messenger cAMP , is activated via the G s protein . This initiates the protein kinase A (PKA) cascade, at the end of which is glycogen phosphorylase (PYG). After phosphorylation it is activated (PYG a ). This releases glucose-1-phosphate from glycogen, which is isomerized to glucose-6-phosphate and can enter into glycolysis . At the same time, PKA also phosphorylates glycogen synthase (GYS a ), which is inactive in its phosphorylated form (GYS b ).
- In the case of insulin, a receptor tyrosine kinase (RTK) is activated. On the way of a complex signal transduction is here u. a. the protein kinase B (PKB) is activated (see lower panel, A). PKB phosphorylates the glycogen synthase kinase 3 , GSK3, which is thereby inactivated. GSK3 is a kinase that phosphorylates the glycogen synthase and thus inactivates it (GYS b ). GSK3 competes with a phosphatase, protein phosphatase 1 (PP1). Because GSK3 can no longer work, there is more and more glycogen synthase in its dephosphorylated form (GYS I, see picture below, B). The PKB also activates a phosphodiesterase, PDE, which hydrolyzes cAMP to AMP. As a result, the signal path for the PKA is also lost.
See also
Web links
Individual evidence
- ↑ Mark E Daly: Acute effects on insulin sensitivity and diurnal metabolic profiles of a high-sucrose compared with a high starch diet . (PDF) In: American Society for Clinical Nutrition (Ed.): Am J Clin Nutr 1998 . No. 67, 1998, pp. 1186-1196. Retrieved February 19, 2011.
- ↑ Guideline for Postprandial Diabetes Management (PDF; 920 kB) International Diabetes Federation . P. 22, 2008. Archived from the original on June 28, 2011. Retrieved July 30, 2011.
- ↑ Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia ( English , PDF; 1.6 MB) In: World Health Organization . who.int. P. 36. 2006. Retrieved February 20, 2011.
- ↑ a b M. A. Rahim, AK Azad Khan, Q. Nahar, SM Ali, A. Hussain: Impaired fasting glucose and impaired glucose tolerance in rural population of Bangladesh. In: Bangladesh Medical Research Council bulletin , Volume 36, Number 2, August 2010, pp. 47-51, PMID 21473200 , ISSN 0377-9238 .
- ↑ Paul-Martin Holterhus u. a .: Diagnostics, therapy, progress monitoring of diabetes mellitus in children and adolescents (PDF), deutsche-diabetes-gesellschaft.de, 2010, p. 18 (accessed on February 20, 2011).
- ^ W. Kerner, J. Brückel: Definition, classification and diagnosis of diabetes mellitus (PDF; 846 kB) DDG. October 1, 2012. Retrieved April 1, 2013.
- ^ Towards Broad Gain Mid-IR Lasers . (PDF; 1.7 MB) Fraunhofer Institute IAF, 2003.
- ↑ Non-invasive blood glucose testing . ( Memento from July 6, 2007 in the Internet Archive ) University of Karlsruhe, Institute for Technology and Information Processing, 2004.
- ↑ In vivo measurement and regulation of the blood sugar level . RWTH Aachen, Chair for Medical Information Technology.
- ↑ Clark Lab | Nanosensors. In: nuweb9.neu.edu. Archived from the original on July 27, 2011 ; Retrieved July 27, 2011 .
- ^ Vince S. Siu, Jing Feng, Patrick W. Flanigan, G. Tayhas R. Palmore, Domenico Pacifici: A “plasmonic cuvette”: dye chemistry coupled to plasmonic interferometry for glucose sensing . In: Nanophotonics . tape 3 , no. 3 , May 6, 2014, p. 125-140 , doi : 10.1515 / nanoph-2013-0057 .
- ↑ J Feng, VS Siu, A Roelke, V Mehta, SY Rhieu, GT Palmore, D Pacifici: Nanoscale plasmonic interferometers for multispectral, high-throughput biochemical sensing . In: Nano Lett. tape 12 , no. 2 , February 8, 2012, p. 602-609 , doi : 10.1021 / nl203325s , PMID 22200183 .
- ↑ a b c Gisela Boeck, Ulrike Bommas-Ebert, Timo Brandenburger: Examination Knowledge Physikum . Georg Thieme, Stuttgart 2009, ISBN 978-3-13-152131-6 , p. 521.
- ↑ Horace Robert Horton: Biochemistry . Pearson Germany, 2008, ISBN 978-3-8273-7312-0 , p. 504.




