insulin
| insulin | ||
|---|---|---|
|
||
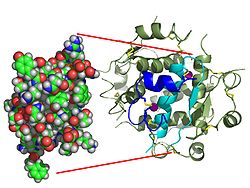
| Two model representations of the insulin molecule. On the left the simple molecule (monomer) as a dome model from which the surface shape emerges. On the right the six-fold molecule (hexamer) as a so-called ribbon model , in which the internal structure becomes clear. In the latter, α-helices are represented by screws and β-sheets by arrows. | ||
|
Existing structural data : 1ai0 , 3ins , 4ins , 6ins , 7ins , 9ins |
||
| Properties of human protein | ||
| Mass / length primary structure | 5.8 kDa / 51 amino acids | |
| Secondary to quaternary structure | Heterodimer (21 + 30 aa) | |
| Precursor | Proinsulin | |
| Identifier | ||
| Gene name | INS | |
| External IDs | ||
| Drug information | ||
| ATC code | A10 AB01 | |
| DrugBank | DB00030 | |
| Occurrence | ||
| Homology family | CLU_140421_1_0 | |
| Parent taxon | Vertebrates | |
| Orthologue | ||
| human | mouse | |
| Entrez | 3630 | 16334 |
| Ensemble | ENSG00000129965 | ENSMUSG00000000215 |
| UniProt | P01308 | Q5EEX1 |
| Refseq (mRNA) | NM_000207 | NM_008387 |
| Refseq (protein) | NP_000198 | NP_032413 |
| Gene locus | Chr 11: 2.14 - 2.14 Mb | Chr 7: 142.49 - 142.49 Mb |
| PubMed search | 3630 |
16334
|
Insulin (other names: insulinum , insulin hormone , islet hormone ) is a proteohormone (polypeptide hormone ) that is vital for all vertebrates and is formed in the β cells of the pancreas . These specialized cells are located in the Islets of Langerhans . The name "insulin" is derived from these islands (from the Latin insula "island"). Insulin is involved in the regulation of the metabolism, especially that of carbohydrates. Insulin lowers blood sugar levels by stimulating cells in the body to take up glucose from the blood .
Function and effect
The concentration of glucose in the blood is regulated by a control circuit made up of two hormones that are released depending on the blood sugar concentration . Insulin is the only hormone that can lower blood sugar levels. Its antagonist is glucagon , the main task of which is to increase blood sugar levels. Even adrenaline , cortisol and thyroid hormones have glucose-enhancing effects.
The blood sugar level rises especially after consuming foods rich in carbohydrates . In response, the β-cells release insulin into the blood . Insulin lowers the blood sugar level by enabling the glucose from the blood plasma and the tissue fluid to pass through the cell membrane into the cell interior by means of its "key function" . The liver and muscle cells in particular can absorb large amounts of glucose in a short period of time and store them in the form of glycogen or break them down for energy production (see glycolysis ).
The hormone also affects other cells, so it has an impact on fat and amino acid metabolism as well as on the potassium balance .
The hormone is an essential factor in the following diseases:
The insulin that circulates in the blood works by binding to insulin receptors.
Insulin receptor
Ultimately, the binding of this hormone triggers to its receptor , a number of kinase - cascades (cascade of phosphorylation of) passing through signal paths can be described.
These signaling pathways cause the blood glucose level to drop
- Promotion of glucose uptake ( GLUT4 - translocation to the cell surface)
- Promotes glucose storage ( glycogen synthesis ) in the liver and muscles
This signal is supported by the activation of glucose consuming pathways. Further supportive measures consist in the suppression of glucose-delivering pathways, for example by breaking down the second messenger cAMP via a phosphodiesterase .
Glucose uptake in muscle tissue
The hormone increases the permeability (permeability) of the cell membrane for glucose in the muscles and fat tissue . It should be noted that it is not the membrane itself that becomes more permeable, but that more carrier proteins for glucose are activated. This carrier protein is GLUT4, a high-affinity, insulin-dependent glucose transporter , which transports the glucose into the cell by facilitated diffusion (passive transport). The following physical properties are relevant for GLUT4: saturable , not activatable or inactivable, i.e. regulation only through insulin-dependent incorporation or expansion.
Glucose uptake and metabolism in the brain
Nerve cells (and erythrocytes ) take glucose-insulin- un depend on. Therefore, when the insulin level increases, the insulin-dependent cells take up more glucose and less is left for the insulin-independent cells.
In general, with hypoglycaemia there is a risk that the glucose-dependent nervous system will be damaged. Insulin, given as a nasal spray in direct contact with the CNS , is being studied for the treatment of Alzheimer's disease .
Build-up and breakdown of adipose tissue
The hormone inhibits lipolysis in adipose tissue and thus the breakdown of fat . Insulin deficiency therefore leads to increased lipolysis with the formation of ketone bodies and the resulting ketosis .
Promote cell growth
Another central function of the peptide hormone insulin is the regulation of cell growth and proliferation by activating the transcription of genes that are of great importance for the control and course of the cell cycle. This effect of insulin is an issue in discussions about insulin preparations .
Tryptophan uptake in the brain
Higher insulin levels have a slightly increasing influence on the absorption of tryptophan in the brain.
Insulin and the regulation of blood sugar levels
One of the most important biological effects of insulin is the rapid acceleration of glucose uptake in muscle and fat cells and regulation of intermediate storage in the liver as part of regulating blood sugar levels :
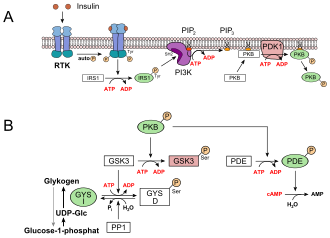
- The carbohydrates ingested with food are stored as glycogen in the liver and muscles . This results in a decrease in the glucose concentration in the blood. Glucose uptake in the liver cells is independent of insulin via GLUT2. A receptor tyrosine kinase (RTK) is activated by insulin , which initiates signal transduction. The insulin receptor substrate 1 (IRS1), the phosphoinositide-3-kinase (PI3K), the second messenger phosphatidylinositol-4,5-bisphosphate (PIP 2 ), the phosphoinositide-dependent kinase-1 (PDK1) and finally the protein kinase are involved B (PKB) (see picture, A). PKB phosphorylates the glycogen synthase kinase 3 , GSK3, which is thereby inactivated. GSK3 is a kinase that phosphorylates and thus inactivates glycogen synthase (GYS b ). GSK3 competes with a phosphatase , protein phosphatase 1 (PP1). Because GSK3 can no longer work, there is more and more glycogen synthase in its dephosphorylated form (GYS a , see figure below, B). The PKB also activates a phosphodiesterase, PDE, which hydrolyzes cAMP to AMP. As a result, the signal path for protein kinase A , which is responsible for the breakdown of glycogen, is also lost.
- Triglyceride synthesis is stimulated in the liver, adipose tissue and muscles under the influence of insulin . In addition to carbohydrates, the substrates for this are lipids taken in with food .
- In the three tissues mentioned, amino acids are increasingly absorbed and used for protein synthesis.
The metabolic and mitogenic effects of insulin are initiated by binding to its receptor on the cell surface of the target tissues, liver, muscle and fat.
- Insulin also induces glycogen synthesis and storage in the liver and muscles, triglyceride synthesis in liver and adipose tissue, and the storage of amino acids in muscles.
- At the same time, insulin inhibits hepatic gluconeogenesis and is therefore one of the most important regulators of glucose metabolism .
Opponent
If the blood sugar level in the body falls below a value of 80 mg / dl, the insulin production is already greatly reduced.
If the blood sugar drops further, various opponents of insulin occur:
The levels of these counter-regulating hormones increase significantly when blood sugar falls below 60 mg / dl.
In type 1 diabetes, the counter-regulation mechanism is often disturbed, which leads to additional problems with hypoglycaemia .
Somatostatin has an inhibitory effect on the secretion of insulin and glucagon because it acts as a general inhibitor in the body.
Effect on potassium levels
Insulin lowers the potassium level in the blood by ensuring that potassium is shifted into the interior of hepatocytes and skeletal muscle cells , i.e. from extracellular to intracellular . It does this by activating the sodium-potassium ATPase with insulin. Insulin preparations are therefore used together with glucose (to prevent hypoglycaemia ) to treat hyperkalemia .
Occurrence
Insulin sequences from more than 100 different species are known. The protein sequences of the respective insulins are similar - they show sequence homology - but are not identical. About the differences in the chemical structure of human insulin compared to the insulins of some mammals as well as information about artificially produced insulin see insulin preparation .
Insulin and evolution : Genotypes that prevented the rapid depletion of energy reserves in hunter-gatherer societies when there was a lack of food, predispose to obesity and type 2 diabetes in today's lifestyle with lack of exercise and excess food .
The extent to which genes influence glucose metabolism and the associated effects of insulin has not yet been fully clarified.
The homologues of insulin in insects are the Drosophila insulin-like peptides .
Education, storage, release and regulation
biosynthesis
The synthesis of the hormone takes place in the β cells of the islets of Langerhans in the pancreas . The genetic information is encoded by only one gene locus in the short arm of chromosome 11 . The gene consists of around 300 nucleotides .
The mRNA is first translated into preproinsulin , which consists of 110 amino acids, on ribosomes located on the rough endoplasmic reticulum (ER) .
The further processing takes place in two steps, after the folding of the molecule by the formation of disulfide bridges , the insulin molecule is formed by splitting off the signal peptide and C-peptide.
|

Processing of preproinsulin into insulin
|
storage
The insulin molecules are in the vesicles of the Golgi apparatus , which is located on the cell membrane of β-cells by zinc - ions to hexamers bound and stored so stabilized (zinc insulin complex).
The ability of insulin molecules to bind zinc has several important effects. Insulin is not yet effective in the form of hexamers and after breaking down into dimers , but only as a single molecule. This property plays an important role in insulin preparations . In the case of fast-acting insulin preparations , the slow disintegration of the molecular assemblies is undesirable and possibilities are sought to accelerate the disintegration. In the case of long-acting insulin preparations, the binding of zinc is specifically strengthened by high zinc concentrations to extend the duration of action . In the development of oral insulin preparations, the zinc bond is used to couple insulin to transport molecules.
distribution

The release of insulin into the blood occurs through exocytosis .
The insulin is released in an oscillating manner . Insulin is released into the bloodstream every three to six minutes . After eating, a biphasic course of insulin secretion can be determined in metabolically healthy people: the first “insulin peak ” peaks after three to five minutes and lasts ten minutes. This is followed by a second phase that lasts as long as the hyperglycemia exists. The first phase consists of the stored insulin molecules, the second phase mainly of newly formed insulin.
The C-peptide is only cut out of the proinsulin by peptidases when the blood sugar level rises and released together with the active insulin and zinc. By detecting C-peptide in serum, endogenous insulin production can be measured. For diabetics, for example, a statement can be made about how much insulin is still being produced by the body, since the synthetic product does not contain a C sequence.
regulation
The main task of insulin, in conjunction with glucagon, is to keep the blood glucose level between certain values. If the blood sugar level falls below a certain value, there is a risk of hypoglycemic coma and death, if it rises above a certain level, there is a risk of damage to blood vessels, kidney corpuscles and other tissues. Together with leptin, however, insulin also seems to have a significant share in energy homeostasis . Not only does leptin act on insulin, but the insulin itself also acts on leptin, more precisely on the absorption of leptin in the brain.
Due to its vital function, the biochemical implementation of the control loop must be robust. Since cells cannot “think”, it can only be that single cells function like a state machine or that few cells work together in such a way that a stimulus is sensibly calculated.
The main stimulus for the release of insulin from the β-cell is the blood sugar level (from 5 mmol glucose / l blood), and this is "measured" directly by the β-cell. This is implemented biochemically with the help of ATP-controlled potassium channels, etc., see #glucose -controlled release mechanism .
The hormones gastrin , secretin , GIP and GLP-1 modify the basic glucose-insulin control circuit by acting on the β-cell. See incretin effect .
GIP is secreted into the blood by K cells , which are located in the mucous membrane of the duodenum and "measure" the glucose in the chyme , and increases the release of insulin by the β cells. The GIP thus acts on the β cells at a time when the glucose from food has not yet reached the blood.
GLP-1 is secreted into the blood by L cells , which are located in the mucous membrane of the ileum and caecum and “measure” the glucose in the chyme, and also increases the release of insulin by the β cells. The majority of the nutrients are already extracted in the jejunum and released into the blood or lymph. There is no absorption of nutrients in the cecum, but mainly fermentation.
In addition, β cells are innervated by both the parasympathetic and sympathetic nervous systems :
The parasympathetic NS is activated in the case of leptin insufficiency (or also in the case of leptin resistance) and generally has a trophotropic effect, i.e. that is, it causes energy to be stored. So it increases insulin delivery. At least in mice, it also acts simultaneously on adipocytes and increases their sensitivity to insulin, while the sensitivity of liver and muscle cells is not affected. Glucose is built into glycogen in liver and muscle cells and stored in this way; de novo lipogenesis and storage of TGs take place in the adipocytes.
The sympathetic NS is activated in leptin insufficiency and has a general ergotropic effect, i. that is, it causes energy consumption to increase. It lowers insulin delivery. The reduction in insulin release seems to counteract increased energy consumption, because the muscle cells can absorb much more glucose from the blood if they have as many GLUT-4 transporters on the surface. So you have to fall back on the already stored energy in the form of glycogen and fatty acids.
Glucose-controlled release mechanism
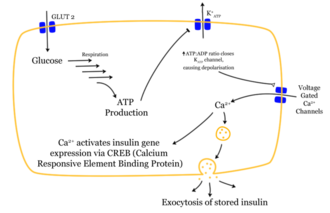
The penetration of a glucose molecule into the β-cell sets a chain of effects in motion. After glucose has entered the cell via the GLUT1 transporter, it is metabolized by glycolysis . The resulting ATP inhibits the outflow of potassium ions ( ATP-sensitive potassium channels ). The greatly reduced potassium outflow leads to depolarization , because the stability of the membrane potential is no longer maintained by the potassium outflow. The depolarized membrane potential causes voltage-dependent calcium channels to open . The influx of calcium ions is the decisive stimulus for the fusion of the insulin-containing vesicles with the cell membrane.
The stored insulin molecules are released by fusing the membranes ( exocytosis ) from the β cells into the extracellular space and further into the bloodstream. The storage hexamers are separated. The level of insulin in the blood increases.
Half-life and degradation
The biological half-life of individual insulin molecules in the bloodstream is around five minutes.
The insulin is absorbed into the cells via some insulin receptors, where it is broken down and thus used up. In the liver and kidneys , insulin is inactivated by insulinase ; insulinase, or more precisely glutathione-insulin transhydrogenase, splits the disulphide bridges between the A and B chains , causing the insulin to split into two parts and become ineffective. The breakdown products are excreted by the kidneys, as is 1.5% of the insulin that is still intact. The short duration of the activity of the insulin shows that the physiological control of the sugar metabolism works very quickly in the healthy body; this speed can not practically be achieved in the treatment of diabetes mellitus .
Insulin as a drug

In the insulin therapy different are insulin preparations used. The most common and oldest method of administration is subcutaneous injection . A number of short-, medium- and long-acting human insulins and insulin analogs are available for this purpose . If these are combined for therapy, special attention must be paid to the different half-lives .
- very fast and short-acting: insulin glulisin, insulin lispro, insulin aspart
- short-acting: normal insulin (= dissolved human insulin)
- Intermediate acting: NPH insulin , biphasic insulin lispro, biphasic insulin aspart
- long-acting: insulin detemir , insulin glargine , insulin degludec
Peroral insulin is ineffective, since the protein chains in the gastrointestinal tract of the body's enzymes are broken down before they can take effect. The extent to which insulins can be encapsulated in nanoparticles is being investigated so that they can be "undigested" introduced into the bloodstream in this way. More recent developments such as preparations for inhalation , which deliver insulin via the respiratory tract, have so far not been able to hold their own on the market.
In the past, insulin was used as part of insulin shock therapy to treat people with mental illness . This method of treatment was practiced, for example, in the biographical film A Beautiful Mind by John Nash . This procedure is no longer practiced.
Insulin abuse
Insulin is on the list of prohibited doping substances because it can be misused for several purposes. Since insulin counteracts the reduced glucose uptake in muscle cells caused by somatropin , it is often used to compensate for its undesirable side effects (see anabolic steroids ). Other uses are to promote the replenishment of glycogen stores in endurance athletes and to help build muscle mass.
The improper self-administration of insulin to excessively lower the blood sugar level leads to the clinical picture of hypoglycaemia factitia .
In March 2008, Newcastle male nurse Colin Norris was sentenced to 30 years imprisonment for murdering four of his patients by injecting high doses of insulin.
Timeline for the history of research
| 1869 | Paul Langerhans discovered the islet cells in the tissue of the pancreas. |
| 1889 | Oskar Minkowski and Josef von Mering removed the pancreas from dogs, thereby triggering diabetes mellitus. Shortly afterwards, the islet cells are suspected to be endocrine (hormone-producing) tissue. |
| 1906 | On June 21, 1906, the German internist Professor Georg Ludwig Zülzer carried out an injection on humans for the first time with a calf pancreatic extract called acomatol, which he had isolated and produced by the Schering company. |
| 1909 | The term insulin , "coming from the islands", appeared for the first time . The Belgian pathologist Jean de Meyer (1878–1934) suggested the name “insulin”, derived from the Latin “insula” for the as yet unknown substance. |
| 1910 | The English physiologist Edward Albert Sharpey-Schafer called the substance from the pancreas lacking in diabetics "insulin". It is not clear from the available sources who coined the name first. |
| 1916 | Nicolae Paulescu succeeded for the first time in obtaining insulin from pancreatic tissue. |
| 1921 | succeeded Frederick Banting and Charles Best , the isolation of insulin from the pancreas of animal fetuses, they called it "isletin". |
| 1922 | Successful use in patients and the start of industrial insulin production in Canada |
| 1923 | Frederick Banting and John James Rickard Macleod received the Nobel Prize in Physiology or Medicine for the discovery of insulin. |
| 1926 | Representation of insulin in crystallized form by John Jacob Abel in America |
| 1928 | Oskar Wintersteiner succeeded in proving that insulin is a protein. |
| 1958 | The Nobel Prize in Chemistry went to Frederick Sanger for his work on the structure of proteins, especially insulin . |
| 1963 | Professor Helmut Zahn and his team succeeded in the world's first chemical synthesis of insulin. |
| 1964 | The Nobel Prize for Chemistry went to Dorothy Hodgkin for her determination of the biochemical structure of important substances such as insulin and vitamin B 12 using X-ray structure analysis . |
| 1971 | the three-dimensional protein structure of insulin by Blundell et al. enlightened. |
| 1982 | succeeded for the first time in producing human insulin in large quantities using genetically modified bacteria. |
| 1996 | Insulin lispro (trade name Humalog ) was the first fast-acting insulin analog. |
| 2000 | Insulin glargine (trade name Lantus ) was the first long-acting insulin analog. |
| 2015 | is the first insulin biosimilar on the market with Abasaglar (insulin glargine). |
Web links
Individual evidence
- ^ Matthias Otto: Analytical Chemistry. John Wiley & Sons, 2011, ISBN 978-3-527-32881-9 , p. 557.
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Biochemistry. 6th edition. Spektrum-Verlag, 2007, ISBN 978-3-8274-1800-5 .
- ↑ S. Craft, LD Baker, TJ Montine et al. : Intranasal insulin therapy for alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial . In: Archives of Neurology . tape 69 , no. 1 , January 2012, p. 29–38 , doi : 10.1001 / archneurol.2011.233 .
- ^ PM Daniel, ER Love, SR Moorhouse, OE Pratt: The effect of insulin upon the influx of tryptophan into the brain of the rabbit. In: J Physiol . Vol 312, March 1981, pp. 551-562, doi: 10.1113 / jphysiol.1981.sp013643
- ↑ "Oh dear, the potassium is 7.2 mmol / l". (PDF) Retrieved October 6, 2018 (lecture at the Medical University of Vienna).
- ↑ So also have man , the chimpanzee , the mouse , the rabbit and the zebrafish : similar, but not identical insulins species sequence alignment of insulin .
- ↑ J. Vander Molen, LM Frisse, SM Fullerton, Y. Qian, L. Del Bosque-Plata, RR Hudson, A. Di Rienzo: Population genetics of CAPN10 and GPR35: implications for the evolution of type 2 diabetes variants. In: American Journal of Human Genetics . Volume 76, Number 4, April 2005, pp. 548-560, doi: 10.1086 / 428784 . PMID 15696418 , PMC 1199293 (free full text).
- ↑ Genes influence glucose metabolism. In: aerzteblatt.de . January 18, 2010, archived from the original on November 26, 2015 ; Retrieved November 25, 2015 .
- ↑ Entry on insulin in Flexikon , a wiki from DocCheck , accessed on November 25, 2015.
- ↑ David Owerbach, Graeme I. Bell, William J. Rutter , Thomas B. Shows: The insulin gene is located on chromosome 11 in humans. In: Nature . 286, 1980, p. 82, doi: 10.1038 / 286082a0 .
- ↑ UniProt P01308
- ↑ UniProt entry
- ↑ a b Helmut Schatz (Ed.): Diabetology compact. 4th edition. 2006, ISBN 3-13-137724-0 .
- ↑ Heiner Laube: Insulin resistance . Pathophysiology , Therapy and Perspectives . Uni-Med, 2001, ISBN 3-89599-541-X .
- ↑ Florian Horn: Biochemistry of humans: the textbook for medical studies . 5th edition. Thieme, Stuttgart 2012, ISBN 978-3-13-130885-6 , p. 351 .
- ↑ Mark E Daly: Acute effects on insulin sensitivity and diurnal metabolic profiles of a high-sucrose compared with a high starch diet . In: Am J Clin Nutr . No. 67 , 1998, pp. 1186–1196 (English, ajcn.org [PDF; accessed February 19, 2011]).
- ↑ Medical student council at the University of Munich: Biochemistry script 2 (PDF)
- ^ Hager's Handbook of Pharmaceutical Practice. P. 552.
- ↑ Yu-Hsin Lin et al. a .: Preparation and Characterization of Nanoparticles Shelled with Chitosan for Oral Insulin Delivery. In: Biomacromolecules . 8 (1), 2007, pp. 146-152. doi: 10.1021 / bm0607776
- ↑ WADA: The Prohibited List 2007 ( Memento from April 21, 2014 in the Internet Archive ) (PDF; 125 kB)
- ↑ Doping News: Insulin ( Memento from June 7, 2007 in the Internet Archive )
- ↑ Tom Chivers: Colin Norris, 'Angel of Death' nurse, convicted. In: The Telegraph . March 2, 2008, accessed September 6, 2016 .
- ↑ Jacek Zajac, Anil Shrestha, Parini Patel, Leonid Poretsky: Principles of Diabetes Mellitus . Ed .: Leonid Poretsky. Springer US, New York 2010, ISBN 978-0-387-09840-1 , chapter The Main Events in the History of Diabetes Mellitus , pp. 3–16 , doi : 10.1007 / 978-0-387-09841-8_1 ( friedmanfellows.com [PDF]).
- ↑ Vivienne Baillie Gerritsen: Protein of the 20th century . In: Protein Spotlight. April 9, 2001.
- ↑ Helmut Zahn, Johannes Meienhofen, Dietrich Brandenburg a. a .: Synthesis of the insulin chains and their combination to form insulin-active preparations. In: Journal of Nature Research B . 18, 1963, pp. 1120-1121 ( online ).
- ↑ TL Blundell, JF Cutfield, SM Cutfield et sl. : Atomic positions in rhombohedral 2-zinc insulin crystals . In: Nature . tape 231 , no. 5304 , June 1971, p. 506-511 , PMID 4932997 .