Insulin in the brain
As a hormone of the energy metabolism, insulin is particularly responsible for the regulation of the peripheral glucose metabolism and the promotion of anabolic processes. It has been known for some time that insulin and the insulin receptor also occur in the central nervous system, but their function there is still not fully understood. Complex effects on neurons and cognition are becoming more and more apparent and are gaining in importance.
Sources of insulin
The insulin content in the brain is between 10 and 100 times higher than in the blood plasma . The amount of insulin in the brain also fluctuates during development: the fetal brain has higher levels of insulin than the brain of an adult.
Peripheral sources of insulin
Most of the insulin in the body is made by the β cells of the pancreas . This insulin circulating in the bloodstream can cross the blood-brain barrier and reach the central nervous system. However, an acutely rising peripheral insulin level has little effect on the insulin content in the brain.
Neural sources of insulin
A small amount of insulin is also synthesized in the brain by pyramidal neurons. Some GABAergic neurons even show a transmitter-like release of insulin. Glial cells, on the other hand, do not seem to produce any insulin. However, the actual role of neuronally synthesized insulin and its contribution to the total amount of insulin in the brain remains controversial.
Insulin and the blood-brain barrier
The blood-brain barrier has special insulin binding sites. Two different functions can be identified: on the one hand the classic receptor function (signal transmission) and on the other hand the transport function. However, it has not yet been clarified whether these are proteins of the same gene origin or unique proteins.
Receptor function
The binding sites that are used for signal transmission affect the cells of the blood-brain barrier (BEC = Brain Endothelial Cells). The transport processes of leptin, tyrosine and azidothymidine (an agent for AIDS treatment) are improved by the action of insulin and the gene expression of P-glycoprotein and the catalytic subunit of the glutamate cysteine ligase are increased. At the choroid plexus, the action of insulin can potentially affect the production of cerebrospinal fluid. In addition, the BEC, especially on the membrane facing the brain, contain the insulysin (IDE = insulin-degrading enzyme) and can thus possibly regulate the insulin effect and insulin transport.
Transport process
The transport of insulin through the blood-brain barrier takes place via a receptor-mediated, active transport mechanism. Since an acute increase in the amount of insulin in the blood has little effect on the insulin content in the brain, the transport is a saturable transport process. Insulin binding is significantly higher in newborns than in adults.
It is noteworthy that the transport of insulin across the blood-brain barrier varies depending on the brain region: the highest permeability is found in the area of the pons, medulla oblongata and the hypothalamus, while the occipital cortex has the lowest permeability. In the area of the thalamus and mesencephalon, the blood-brain barrier is even completely impermeable to insulin.
The transport process is also influenced by a number of factors: starvation, obesity, iron status, nitric oxide levels and glucocorticoids. While the glucocorticoids reduce transport, nitric oxide (NO) has different effects: NO, which was produced by nNOS, inhibits insulin transport; However, NO, which was produced by eNOS or iNOS, stimulates the transport process. Since nitric oxide itself does not differ from one another, one of the two pathways will exert its effect indirectly through other cells of the neurovascular unit.
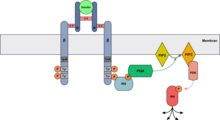
Neuronal insulin receptor and signaling pathways
Distribution and localization of the insulin receptor
Distribution in the brain
The distribution density of the neuronal insulin receptor (nIR) in the brain can vary between the different brain regions by 5 to 10 times. The distribution is independent of vascularization and cell density, but is particularly high in dendritic fields with strong synaptic input. The latter indicates a possible connection between neuronal activity and the distribution density of the nIR. Regions with a high nIR distribution density are: neocortex, hippocampus, olfactory bulb, hypothalamus and cerebellum.
During the development from the embryonic to the adult brain, the nIR density is subject to constant change. Some brain regions such as B. striatum and thalamus show a high nIR density during the development phase, which later decreases in the adult brain.
Distribution at the cellular level
The nIR is primarily localized in the pre- and postsynaptic membranes of the neurons. Correspondingly, the IRS (e.g. IRSp58 or IRSp53; see below: Signaling pathways) are particularly concentrated in the synapses of the neocortex, hippocampus and cerebellum. The synapses are therefore an important point of attack for insulin signaling in the brain.
In addition, the nIR in the cell membrane only react to insulin if they are outside of ganglioside GM1 rafts. Within these membrane sections, the nIR are inhibited by a high level of tyrosine phosphatase activity. A changed ratio of cholesterol and sphingolipids in the spinous processes (dendritic spines) also enables the modification of receptor activities including the nIR.
Structure and differentiation of the insulin receptors
The insulin receptor (IR) belongs to the family of receptor tyrosine kinases. It is a tetrameric glycoprotein, consisting of two α-subunits and two β-subunits which are held together by disulfide bonds. The α-subunits are completely extracellular and bind insulin as well as IGF-1 with a lower affinity. The β subunits have an extracellular, a transmembrane and an intracellular domain.
Basically, the neuronal IR (also IR-A) and the peripheral IR (also IR-B) have identical pharmacological and kinetic properties. The nIR, however, has a lower glycosylation and thus differs from the peripheral IR in terms of its carbohydrate composition and molecular size. Due to alternative splicing, the nIR lacks exon 11. There are also differences in the regulation by insulin: the peripheral IR is downregulated by insulin (the binding of another insulin to the receptor dimer releases the first one), but the nIR is not subject to this downregulation. Most important, however, is the distinction between the two IRs based on their function: while the peripheral IR for its rapid metabolic effects, v. a. through the insertion of glucose transporters, the nIR has a very complex effect on the neurons and causes both short and long-term changes (see below: Physiological functions). It is noteworthy, however, that both insulin receptors are found in the central nervous system. In addition to the neuron-specific nIR, the peripheral IR occurs in low density in some glial cells.
Signaling pathways
The neural insulin receptor works in principle, just like the peripheral IR, mainly via the two PI3K / Akt and Ras / MAPK signaling pathways. The actual downstream signal can, however, differ even between individual neurons depending on the availability of the downstream substrates. Other signal paths and also cross-talking between different signal cascades have so far been little researched, but will certainly gain in importance in the future.
Start of signal transduction
The binding of insulin to the α-subunit leads to the activation of the tyrosine kinase activity of the β-subunit, which initially autophosphorylates its own tyrosine residues. The β subunit then also phosphorylates the tyrosine residues of an insulin receptor substrate (IRS). The various signal paths then originate from the IRS. The IRS-1 and IRS-2 mediate most of the pleiotropic effects in the various cells.
PI3K / act path
The PI3K / Akt signaling pathway begins with the binding of the p85-SH2 domain of the PI3K to the phosphorylated tyrosine of the activated IRS. Activated by this binding, the p110 subunit of the PI3K phosphorylates the membrane-bound PIP 2 to PIP 3 . The modified membrane phospholipid binds the PDK (phosphoinositide-dependent kinase), which in turn activates Akt (PKB) through phosphorylation. Further diverse effects then emanate from the act.
Ras / MAPK way
The Ras / MAPK signaling pathway begins with the binding of Grb2 with its SH2 domain to the phosphorylated tyrosine of the activated IRS. The SOS, in turn, binds to the bound Grb2 and then activates Ras by exchanging GDP for GTP. The active Ras binds and activates Raf-1 and starts the kinase cascade with the phosphorylation of MEK and the subsequent phosphorylation of ERK. The activated ERK reaches the cell nucleus and influences gene expression there via transcription factors.
PKC / NF kB path
The expression of the P-glycoprotein in the BEC (see above: insulin and blood-brain barrier) is influenced by the PKC / NF-κB signaling pathway, which is atypical for the insulin receptors. Many overlaps in the mechanisms of the PKC and PI3K pathways such as B. the activation of PKC by PDK indicate further cross-talking. It is therefore wrong to view the signal pathways as independent and isolated from one another.
Physiological functions
Glucose metabolism
Central glucose metabolism
The glucose metabolism in the central nervous system is usually considered to be insulin-independent, with the glucose transporters GLUT-3 for neurons and GLUT-1 for astrocytes. However, some neurons e.g. B. in the hippocampus the insulin-dependent GLUT-4 overlapping with the insulin receptor. Also in the cerebellum, insulin influences the expression of GLUT-4 in some cells.
Another mechanism by which insulin can influence the glucose balance in the brain is the inhibition of neuronal adrenaline uptake. The subsequent activation of glial β-adrenoreceptors leads to glucose secretion from the glycogen supply of the astrocytes.
Peripheral glucose metabolism
The action of insulin in the hypothalamus can also affect and suppress glucose production in the liver. Deficient insulin signaling in the hypothalamus also leads to desensitization of the liver to circulating insulin and thus to increased gluconeogenesis.
Neuromodulatory Effects
Effect on rate of fire and membrane potential
The effect of insulin on the rate of fire and the membrane potential of neurons is very complex and can vary greatly from cell to cell. In general, however, insulin shows a stimulatory effect on the Na + / K + -ATPase of the brain.
In the cortex of the insula, insulin has a negative effect on the threshold potential via the PI3K pathway and thus increases the rate of fire. In the olfactory bulb, the action of insulin can acutely suppress the outward current of voltage-dependent Kv 1.3 channels through phosphorylation. Hypothalamic neurons are hyperpolarized via the activation of ATP-dependent K + channels. Depending on the amount and exposure time, insulin has an inhibitory or promoting effect on GABA-induced currents, including in the cerebellum.
One effect on the release of transmitter vesicles is the intracellular increase in the Ca 2+ level caused by insulin. It is open, however, whether the insulin-sensitive Ca 2+ store differs from other Ca 2+ stores in the neuron.
Effect on receptors
The glutamatergic transmission is influenced by the phosphorylation of the NR2A and NR2B subunits of the NMDA receptors. The consequence is a potentiation of the receptor responses. By influencing exocytosis, insulin can also promote the translocation of NMDA receptors into the cell membrane. The developmental stage of the brain also has an effect on insulin signaling: while in an embryonic brain the development of silent synapses into functional synapses is promoted by upregulation of the AMPA receptors, the action of insulin in the adult brain promotes clathrin-dependent endocytosis of the AMPA receptors and thus the LTD (long-term depression).
The GABAergic transmission is also influenced by the translocation of GABA A receptors to the plasma membrane. In addition, insulin also increases the expression of the GABA A receptors in postsynaptic membranes and thus the amplitudes of the mIPSCs (miniature inhibitory postsynaptic current). The distribution density of the GABA A receptors on the postsynaptic membrane is also regulated by Akt-dependent phosphorylation.
Other neuromodulatory effects
Insulin signaling also has a relatively diffuse effect on the monoaminergic system. For example, insulin can increase the expression of the dopamine transporter via the PI3K signaling pathway and reduce the activity of monoamine oxidase, which degrades serotonin and dopamine. The rate of fluctuation of adrenaline and noradrenaline can also be increased by inhibiting their resumption. In the hypothalamus, the α2-adrenergic receptors are selectively downregulated by insulin.
Other effects include stimulating the uptake of amino acids by neurons for neurotransmitter synthesis or increasing the phosphatidylinositol content in the membrane of hippocampal neurons. The latter is another way by which insulin can affect receptor-mediated signal transduction.
Neurotrophic Effects
proliferation
The proliferation and differentiation of multipotent neuronal stem cells are apparently also under the influence of insulin-induced signal transduction. Insulin withdrawal leads to non-apoptic, autophagic cell death and the lack of IRS-2 also impairs neuronal proliferation. Conversely, insulin increases the activity of ornithine decarboxylase, which is an indicator of growth and differentiation processes.
differentiation
During cell differentiation in the developing brain, levels of insulin and insulin receptors increase. The associated change in the rate of binding of insulin to the plasma membrane corresponds to the changes in brain growth. It reaches its peak shortly after birth and then decreases again to adulthood. Especially with these developmental fluctuations, the endogenously synthesized insulin may be of importance. Insulin signaling can also act as an integrating factor that coordinates the speed of neural differentiation with changes in the food supply and thus plays an important coordinative role.
Axon growth
Endogenous insulin also plays an important role in axon growth and outperforms peripheral insulin in its effectiveness. Insulin acts in several important places: the correct distribution of the neurofilaments in the axons and the entire neuron morphology are dependent on the functioning MAPK cascade. PI3K / mTOR promotes the outgrowth of axons (e.g. in cerebellar granule cells) and increases tau synthesis. The tau protein affects the growth and polarity of the axon. Tubulin synthesis is promoted by increased expression of the mRNA and its stabilization. Insulin and IGF-2 are also necessary for NGF to bind to its receptor. The insulin receptor is also needed to find the axonal path.
Neuroprotective effects
The signaling pathways triggered by insulin also have a neuroprotective effect. However, it is important that some of these effects are partially modified by astrocytes.
Against apoptosis
The anti-apoptic effects of insulin are exerted through many different signaling pathways. Via PI3K / Akt, insulin prevents the Bcl-2 reduction, caspase-3 activation, DNA fragmentation and causes the inhibition of GSK-3β. Protein synthesis is also maintained through the action of mTOR on the ribosomal protein P70SK. In the case of ascorbate / Fe 2+ -induced cell death, the MAPK cascade proves to be a protective path.
On the other hand, withdrawal of insulin leads to an increased rate of apoptosis in the hippocampus and hypothalamus. During development, the granule cells of the cerebellum are particularly affected. There is also a redistribution of regulatory proteins between the intracellular compartments such as B. membrane bound Grf1 or reduction of mitochondrial Ras.
Against oxidative stress
The accumulations of GABA and glutamate that arise during oxidative stress due to reduced reuptake can be reduced again by the action of insulin. The glucose uptake of the attacked GLUT-3 and its further processing into pyruvate can also be stimulated again by insulin, so that the ATP deficiency and the increased extracellular ADP are counteracted.
The PI3K / Akt / mTOR pathway contributes to restoring the redox balance by translocating Nrf2 into the cell nucleus. The resulting increased activity of the glutamate cysteine ligase stabilizes the GSH / GSSG level. The parallel increase in uric acid also has an antioxidant benefit by stabilizing ascorbic acid.
Against ischemia
Insulin protects neurons from hypoxia-induced necrosis and also from damage caused by decreased glucose intake. However, this protective function is not equally pronounced in all brain regions: In the dentate gyrus of the hippocampus, insulin develops a better protective function than in the regions CA1 and CA3. The Ca 2+ homeostasis, which is disturbed by hypoglycaemia, can be stabilized again by high doses of insulin. The insulin-related increase in the extracellular GABA level leads to the inhibition of neurons. The resulting reduced glucose consumption protects against ischemic damage while at the same time preventing lactic acidosis. The stimulating effect of insulin on the Na + / K + -ATPase turns out to be protective in the same way. The reduction in extracellular K + reduces the neural rate of fire and thus also the cell's energy requirements. In addition, the decreased intracellular Na + level prevents the accumulation of water in the neuron and thus acts against post-ischemic edema.
Memory and Learning: Effects on Synaptic Transmission
Learning processes influence the IR expression in the hippocampus. While IR expression increases in the CA1 region, it decreases in CA3. However, the overall level of insulin receptors decreases. Since the level of IRS-1 and Akt as well as the activity of ERK1 / 2 increase as a result of the learning process, the decrease in insulin receptors is due to the initial increase in IR activity.
Learning and cognition are mainly influenced by the neuromodulatory effects of insulin. Of particular importance here is the effect on glutamaterge and GABAergic transmission as well as the participation in processes of synaptic plasticity such as LTP and LTD. The metabolic effects on the hippocampal neurons, some of which express GLUT-4, can also be added here.
Regulation of food intake and body weight
Food intake is influenced in the hypothalamus by the action of insulin on neurons of the Ncl. arcuatus. Insulin inhibits the expression of the orexigenic neuropeptides NPY (neuropeptide Y) and AgRP (agouti-related protein) and stimulates the expression of the anorexigenic neuropeptides POMC (proopiomelanocortin) and CaRT (cocaine and amphetamine-regulated transcript). Together, these increase the activity of α-MSH in neurons of the Ncl. paraventricularis, which inhibits food intake. In orexigenic neurons, signal transmission takes place at least partially via the PI3K pathway, which leads to hyperpolarization of the neuron by influencing ATP-dependent potassium channels.
In contrast, when insulin signaling is disturbed, JNK is activated, which in turn phosphorylates IRS-1 and thus promotes feedback inhibition of the insulin receptor. The unchanged activity of the orexigenic neurons ultimately leads to weight gain.
Angiogenesis in the central nervous system
Angiogenesis in the CNS is controlled by a large number of factors and involves many cell types in the central nervous system. A key factor is the HIF (hypoxia-induced factor) activated by hypoxia and acting as a transcription factor. Insulin has an activating effect on the HIF via both PI3K and MAPK signaling pathways. Numerous genes such as B. EPO, VEGF, GLUT1-3 are thus regulated by hypoxia and also by insulin.
Pathological roles
Insulin and Alzheimer's disease
Disturbed insulin signaling and a reduced reactivity of the insulin receptors to insulin are common features of brains with Alzheimer's disease. Such insulin resistance can occur globally due to type 2 diabetes mellitus or limited to the central nervous system. However, there are other factors that contribute to impaired signal transduction: the soluble amyloid-β-oligomers (AβO), which contribute to memory loss in Alzheimer's disease as synaptotoxins, trigger mechanisms in the brain similar to those of peripheral type 2 diabetes mellitus. By activating the TNF-α / JNK signaling pathway, IRS-1 and Akt are phosphorylated and inactivated. Furthermore, the internalization and redistribution of the insulin receptors is initiated.
The defective insulin signaling in turn promotes β-amyloid synthesis and anti-apoptic signaling pathways are inhibited. The dysregulated NMDA receptor signaling and the formation of tau aggregates also interact with the disturbed insulin signaling pathways.
Parkinson's disease
Parkinson's disease is primarily characterized by the degeneration of the dopaminergic neurons of the substantia nigra, pars compacta in the mesencephalon. In the healthy organism, this brain region also has a high density of insulin receptors and IGF-1 seems to be an effective protective factor against the loss of dopaminergic neurons.
Affective disorders
Several studies associate diabetes mellitus with depressive disorders. The exact mechanisms are currently completely unclear, but there are possible links to the insulin-related influence on the monoaminergic systems or indirect interactions such as inflammation and increased cytokine production.
References
- WA Banks, JB Owen, MA Erickson: Insulin in the brain: There and back again. In: Pharmacology & Therapeutics. Volume 136, 2012, pp. 82-93.
- E. Blázquez, E. Velázquez, V. Hurtado-Carneiro, JM Ruiz-Albusac: Insulin in the Brain: Its Pathophysiological Implications for States Related with Central Insulin Resistance, Type 2 Diabetes and Alzheimer's Disease. In: Frontiers in Endocrinology. 5, 2014, p. 161.
- FG De Felice, MV Lourenco, ST Ferreira: How does brain insulin resistance develop in Alzheimer's disease? In: Alzheimer's & Dementia. Volume 10, Suppl. 1, 2014, pp. S26-S32.
- AI Duarte, PI Moreira, CR Oliveira: Insulin in Central Nervous System: More than Just a Peripheral Hormone. In: Journal of Aging Research. 2012, pp. 1–21.
- R. Ghasemi, A. Haeri, L. Dargahi, Z. Mohamed, A. Ahmadiani: Insulin in the Brain: Sources, Localization and Functions. In: Molecular Neurobiology. Volume 47, 2013, pp. 145-171.
- M. Gralle: The neuronal insulin receptor in its environment. In: Journal of Neurochemistry. Volume 140, 2017, pp. 359-367.
- SM Gray, RI Meijer, EJ Barrett: Insulin Regulates Brain Function, but How Does It Get There? In: Diabetes. Volume 63, 2014, pp. 3992-3997.
- A. Kleinridders, HA Ferris, W. Cai, CR Kahn: Insulin Action in Brain Regulates Systemic Metabolism and Brain Function. In: Diabetes. Volume 63, 2014, pp. 2232-2243.
- S. Kullmann, M. Heni, A. Fritsche, H. Preissl: Insulin Action in the Human Brain: Evidence from Neuroimaging Studies. In: Journal of Neuroendocrinology. Volume 27, 2015, pp. 419-423.
- L. Plum, M. Schubert, JC Brüning: The role of insulin receptor signaling in the brain. In: Trends in Endocrinology & Metabolism. Volume 16, 2005, pp. 59-65.
- M. Ramalingam, S.-J. Kim: Mechanisms of action of brain insulin against neurodegenerative diseases. In: Journal of Neural Transmission. Volume 121, 2014, pp. 611-626.
- Y. Zeng, L. Zhang, Z. Hu: Cerebral insulin, insulin signaling pathway, and brain angiogenesis. In: Neurological Sciences. Volume 37, 2016, pp. 9-16.