adrenaline
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| Structural formula of ( R ) - (-) - adrenaline | |||||||||||||
| General | |||||||||||||
| Non-proprietary name | Epinephrine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 9 H 13 NO 3 | ||||||||||||
| Brief description |
white solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | 183,20 g · mol -1 | ||||||||||||
| Melting point |
211-212 ° C |
||||||||||||
| pK s value |
8.6 |
||||||||||||
| solubility |
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Adrenaline (formed 1901 from latin ad on 'and reindeer , kidney') or epinephrine (1900 formed from ancient Greek ἐπί epi on 'and νεφρός nephros , kidney') is a adrenal medulla formed hormone belonging to the group of catecholamines belongs. Adrenaline also occurs in the central nervous system , where it is present as a neurotransmitter in adrenergic nerve cells . Adrenaline mediates its effects by activating G-protein-coupled receptors , the adrenoceptors . The more effective form L-adrenaline came on the market as suprarenine (from the Latin supra , 'over') before 1919 .
Once released into the blood, adrenaline mediates an increase in the heart rate , an increase in blood pressure caused by constriction of blood vessels and a dilation of the bronchioles . The hormone also causes a rapid supply of energy through fat breakdown ( lipolysis ) and the release and biosynthesis of glucose . It regulates the blood circulation (centralization) and the stomach - intestinal -Tätigkeit (inhibition). As a stress hormone , it is involved in the " fight-or-flight response ".
Definition of terms
A frequently used name for adrenaline (originally a brand name) is epinephrine ( INN ) ( ancient Greek ἐπί epí 'on' and νεφρός nephrós 'kidney').
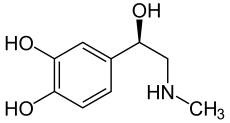
Adrenaline has a stereocenter, so there are two enantiomers . If the name "adrenaline" is not identified by a descriptor , the naturally occurring ( R ) - (-) - adrenaline is meant. ( S ) - (+) - Adrenaline, on the other hand, has practically no meaning.
Discovery story
The first evidence of a substance occurring in the adrenal medulla and released from there into the bloodstream, which could be stained with iron (III) chloride , was found in 1856 by the French physiologist Alfred Vulpian . The practicing physician George Oliver and the physiologist Edward Albert Schäfer established in 1893/94 that this substance must have extraordinary pharmacological properties . The same was achieved in 1894 by the Krakow physiologist Napoleon Cybulski with his assistant Władysław Szymonowicz . In 1896 the ophthalmologist William Bates published his observations.
John Jacob Abel presented the still impure substance in 1897 and 1900 respectively and gave it the name "Epinephrine". Inspired by his work, Jokichi Takamine and Thomas Bell Aldrich (1861–1938) isolated these in 1901 and had them marketed by Parke, Davis & Co. under the name “Adrenalin”. Although Abel's epinephrine later turned out to be an artifact of isolation, the name epinephrine is still used as a synonym for adrenaline.
In 1904, the formula and chemical synthesis followed by Stolz in Höchst . In 1908 Fritz Flaecher (1876–1938) succeeded in separating the racemate into the two enantiomers , with the more effective L-form being brought onto the market under the name Suprarenin . In 1919 Reinhard von den Velden (1880–1941) carried out the first intracardiac adrenaline injection.
Adrenaline was the first hormone to be manufactured and its structure determined. Further adrenaline research led to the body's other two catecholamines, noradrenaline and dopamine .
Biosynthesis and degradation
biosynthesis
The biosynthesis of adrenaline starts from the α- amino acids L - tyrosine or L - phenylalanine . These are hydroxylated to L-DOPA . After decarboxylation to biologically active dopamine, enantioselective hydroxylation to noradrenaline takes place , which can also be released from the adrenal medulla and also acts as a transmitter in sympathetic neurons. The N -methylation of noradrenaline ultimately supplies the adrenaline. The normal concentration of adrenaline in the blood is below 100 ng / l (around 500 pmol / l).
Regulation of biosynthesis
The biosynthesis and release of adrenaline can be controlled by nerve stimuli, hormones or drugs. Nerve irritation promotes the conversion of L- tyrosine to L- dopa and of dopamine to norepinephrine. Cortisol , the hormone in the adrenal cortex , promotes the subsequent conversion of noradrenaline to adrenaline.
Adrenaline production can also be regulated through a negative feedback mechanism. Rising adrenaline levels are negatively fed back to the L- tyrosine formation , so with increased adrenaline levels the L- tyrosine formation is slowed down.
Dismantling
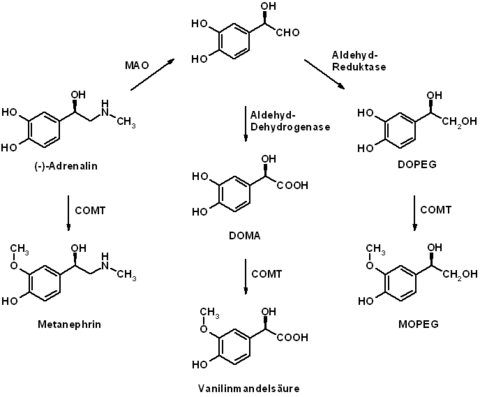
After its release, adrenaline is broken down again relatively quickly. The plasma half-life of adrenaline is only one to three minutes when administered intravenously. The enzymes catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO) are particularly involved in the breakdown of adrenaline . The primary degradation product metanephrine (see metanephrine ) formed by O-methylation (COMT) no longer has any significant biological activity. Metabolism to vanillin-mandelic acid and 3-methoxy-4-hydroxyphenylethylene glycol (MOPEG) is possible through further, in particular oxidative, metabolic processes involving monoamine oxidase . These metabolic products are excreted in the urine in conjugated (e.g. as sulfates ) and unconjugated form . The reliable qualitative and quantitative detection of all metabolites is achieved through the coupling of different chromatographic methods.
Effects
Adrenaline is a stress hormone and as such creates the prerequisites for the rapid provision of energy reserves that are supposed to ensure survival in dangerous situations ( fight or flight ). These effects are mediated at the subcellular level through activation of the G-protein- coupled adrenergic receptors.
Cardiovascular system
Of particular importance is the effect of adrenaline on the cardiovascular system. This includes u. a. the increase in the central blood volume, which occurs through contraction of small blood vessels , especially in the skin and in the kidneys , via the activation of α 1 -adrenoceptors . At the same time, a β 2 -adrenoceptor -mediated expansion of central and muscle supplying blood vessels is observed.
The activation of β 1 -adrenoceptors leads to an increased heart rate (positive chronotropic effect), accelerated conduction of excitation (positive dromotropic effect), increased contractility (positive inotropic effect) and a lowering of the stimulus threshold (positive bathmotropic effect). These effects improve cardiac output and, by constricting small blood vessels, help increase blood pressure. After pretreatment with alpha blockers , however, adrenaline leads to a paradoxical, therapeutically used lowering of blood pressure ( adrenaline reversal ). Even very low doses of adrenaline (<0.1 µg / kg) can cause a slight decrease in blood pressure, which is explained by the selective activation of β 2 -adrenoceptors in the blood vessels.
Chronically elevated adrenaline levels have been associated with hypertrophy of the heart.
Smooth muscles, breathing, gastrointestinal tract, urinary bladder
In addition to the above-mentioned function on the cardiovascular system, the increase in breathing and a temporary inactivation of unnecessary processes, e.g. B. the digestion, in the context of the stress hormone function of the adrenaline of importance. By activating β-adrenoceptors, adrenaline leads to relaxation of the smooth muscles . This has, for example, a immobilization of the gastrointestinal tract (inhibition of peristalsis ) and an expansion of the bronchial tubes to facilitate breathing (β 2 -adrenoceptors). Adrenaline can also cause relaxation of the uterus in pregnant women via β 2 -adrenoceptors . On the other hand, adrenaline can mediate smooth muscle contraction in organs that predominantly express α 1 -adrenoceptors. For example, adrenaline causes the urinary bladder sphincter to contract .
Mobilization of energy reserves
The release of adrenaline from the adrenal gland leads to the mobilization of the body's own energy sources by increasing fat breakdown (lipolysis). This lipolysis is catalyzed by a β-adrenoceptor-mediated (predominantly β 3 -adrenoceptors) activation of the hormone-sensitive lipase . An increase in the adrenaline level also leads to the release and formation of new glucose and thus to an increase in the blood sugar level (β 2 -adrenoceptors). This effect is enhanced by the α 2 -adrenoceptor- mediated inhibition of insulin production and the β-adrenoceptor-mediated release of glucagon . Adrenaline increases the uptake of glucose in muscles. Adrenaline also leads to an increase in energy expenditure (predominantly β 2 -adrenoceptors).
Central nervous system
Observed central nervous effects as a stress hormone are viewed as reflective, since adrenaline formed in the adrenal gland can not cross the blood-brain barrier . Regardless of this, locally produced adrenaline could be detected as a neurotransmitter in some neurons of the central nervous system. These neurons occur particularly in the area of reticularis superficialis ventrolateralis . The exact function of these adrenergic neurons is not yet known, but a role in central blood pressure regulation and the baroreceptor reflex is being discussed. The central nervous system perceives the stressor, then the hypothalamus becomes active and activates the sympatheticus. Its stimulating effect on the adrenal medulla causes it to release adrenaline and noradrenaline.
Other effects
As a result of the release of adrenaline or a local application of adrenaline, sweat production , goose bumps (pilomotor reflex) and pupillary dilation ( mydriasis ) can be observed. You also get a dry mouth. Adrenaline is also involved in blood clotting and fibrinolysis .
chemistry
| Enantiomers of adrenaline | ||
| Surname | ( R ) -adrenaline | ( S ) -adrenaline |
| Structural formula |

|
|
| other names |
L-adrenaline (-) - adrenaline |
D-adrenaline (+) - adrenaline |
| ( RS ) -adrenaline DL -adrenaline (±) -adrenaline |
||
| CAS number | 51-43-4 | 150-05-0 |
| 329-65-7 (racemate) | ||
| EC number | 200-098-7 | 205-752-5 |
| 206-347-6 (racemate) | ||
| ECHA info card | 100,000,090 | 100.005.230 |
| PubChem | 5816 | 247704 |
| 838 (racemate) | ||
| Wikidata | Q132621 | Q27074317 |
| Q7279006 (racemate) | ||

Adrenaline (chemically: ( R ) -1- (3,4-dihydroxyphenyl) -2- ( N -methylamino) ethanol) belongs to the group of catecholamines , which also includes noradrenaline and dopamine. The effective form ( eutomer ) of adrenaline has stereochemically an ( R ) configuration [( R ) -adrenaline or (-) - adrenaline]. ( R ) -Adrenaline is about 20 to 50 times more effective than ( S ) -adrenaline.
synthesis
Several processes are described in the literature for the synthesis of adrenaline. The classic synthesis process comprises three steps: Pyrocatechol ( 1 ) is acylated with chloroacetic acid chloride ( 2 ) to give 3,4-dihydroxy-ω-chloroacetophenone ( 3 ) . The reaction corresponds indirectly to the Friedel-Crafts acylation , the preferred route nevertheless leads via the ester intermediate and thus includes a Fries rearrangement . Amination of the chloroacetophenone with methylamine gives the adrenalone ( 4 ); the subsequent reduction yields racemic adrenaline ( 5 ). The racemate resolution is possible with the help of (2 R , 3 R ) - tartaric acid .
Alternatively, 3,4-dimethoxybenzaldehyde can also be reacted with hydrocyanic acid to form cyanohydrin , the oxidation of which then yields a nitriloketone. Catalytic reduction produces an amino ketone , whose gentle N - methylation then yields the secondary amine . Adrenaline is then obtained through hydrolysis of the phenyl ether functions , reduction and racemate resolution.
Commercially available forms of adrenaline are also hydrogen tartrate and hydrochloride .
stability
Like all catecholamines, adrenaline is sensitive to oxidation. One of the products of oxidation of adrenaline is adrenochrome . Silver (I) oxide (Ag 2 O) can be used for the oxidation . The oxidation of adrenaline can also be catalyzed in aqueous solution by traces of iron and iodide ions . Antioxidants such as B. Ascorbic acid and sodium metabisulphite can slow down the formation of adrenochrome. The rate of oxidation also depends on the pH of the solution. A slightly acidic pH value is considered the optimum stability.
Adrenaline as a drug
application areas
In medicine , adrenaline is mainly used as an emergency medication for cardiopulmonary resuscitation in cardiac arrest and anaphylactic shock . It is available in various dosage forms and requires a prescription .
Emergency medicine
For use in emergency medicine , adrenaline is administered intravenously , alternatively intraosseously or, very rarely, intracardially . In the current recommendations of the European Resuscitation Council , the administration of adrenaline during resuscitation is recommended as the standard. In a large placebo-controlled study, the use of adrenaline during resuscitation outside the hospital was shown to improve survival, but this was also associated with a higher number of neurological damage.
Another main area of application of adrenaline in medicine is circulatory shock , for example in anaphylactic reactions or sepsis . Treatment of anaphylactic reactions and anaphylactic shock is via intramuscular administration of adrenaline. If there is no improvement in condition with intramuscular administration during acute shock , adrenaline can also be administered intravenously titrated. For patients with severe allergic reactions in the past (. Eg impending suffocation due to swelling of the glottis ( glottis )) are adrenaline-filled syringes are available, which then can be applied by the person concerned after allergen exposure with early symptoms themselves.
For use in cardiopulmonary resuscitation and in shock, the centralizing effects of adrenaline are in the foreground. Activation of α 1 -adrenoceptors constricts small blood vessels in the skin and kidneys, while large central blood vessels are widened. In this way, adrenaline is said to increase coronary and cerebral perfusion pressure.
Respiratory diseases
Adrenaline is available as an inhalation solution (InfectoKrupp Inhal ® ) for use as additional medication in acute subglottic laryngitis (" pseudo-croup ") . Up to 2002, inhalation preparations containing adrenaline were also approved for the acute treatment of bronchial asthma in Germany. However, when the CFC ban came into force , these were withdrawn from the market. The inhalative use of other adrenaline preparations for the acute treatment of asthmatic complaints is therefore outside of the drug approval and corresponds to off-label use .
The use of adrenaline in respiratory diseases is based on its bronchial relaxing effect, which is mediated by activation of β 2 -adrenoceptors. However, systemic side effects after absorption must be accepted.
Local vasoconstriction
Adrenaline can also be used to narrow the blood vessels locally in the event of bleeding. The vasoconstricting effect is also used to close cuts in boxing . This vasoconstrictive effect is based on the activation of α 1 -adrenoceptors of small blood vessels in the skin and muscle tissue and their subsequent narrowing.
Adrenaline is also used as a vasoconstrictive additive to local anesthetics to slow down their removal and thus extend their duration of action.
Antidote
Adrenaline is the drug of second choice for beta blocker poisoning and can be used when no specific beta agonist is available. However, there is also no drug approval ( off-label use ) for this emergency use .
Side effects
The side effects of adrenaline largely correspond to its main effects and are due to its importance as a stress hormone. Adrenaline leads to a contraction of small blood vessels, especially the skin and kidneys, combined with an increase in blood pressure and, especially when applied locally, occasional necrosis . With systemic use, cardiac side effects such as B. heart failure, angina pectoris attacks , myocardial infarction, tachycardiac arrhythmias, up to ventricular fibrillation and cardiac arrest in the foreground. Therefore its application is partly controversial. The systemic use of adrenaline can also lead to an increase in the blood sugar level ( hyperglycaemia ), a decrease in the potassium level ( hypokalaemia ), metabolic acidosis and a decrease in the magnesium concentration ( hypomagnesaemia ). Furthermore, mydriasis, micturition difficulties , salivation, sweating with simultaneous feeling of cold in the extremities, nausea , vomiting , dizziness and headache can be observed. The psychological side effects of the use of adrenaline can include restlessness, nervousness, anxiety, hallucinations, convulsions and even psychoses.
Interactions
Some inhalation anesthetics, which sensitize the heart to catecholamines, lead to an increased effect of adrenaline on the heart and thus to an increased risk of heart failure, angina pectoris attacks, myocardial infarction and tachycardiac arrhythmias.
The effects and side effects of adrenaline can also be increased by inhibiting the depletion of adrenaline or by increasing the release of (nor-) adrenaline. This can be observed particularly with simultaneous use of MAO inhibitors , levodopa, L-thyroxine , theophylline , tricyclic antidepressants and reserpine .
In turn, adrenaline inhibits the antihypertensive effects of alpha blockers and the cardiac effects of beta blockers. Since adrenaline increases blood sugar levels, the effectiveness of oral antidiabetic drugs is reduced.
dosage
Adrenaline is given as a solution intravenously. The concentration in an ampoule is typically 1 mg / ml (also referred to as adrenaline solution 1: 1,000 or adrenaline solution 0.1%). Depending on the area of application, it is common to dilute in a ratio of 1:10 with 0.9% sodium chloride solution (then referred to as adrenaline solution 1: 10,000 or adrenaline solution 0.01%). The resuscitation dose is 1 mg every 3–5 minutes. In intensive care medicine and for the treatment of a low output syndrome , a dosage of 2–20 µg / min is used in adults.
Trade names according to dosage form
Ampoules (solution for injection)
- Suprareness (D)
- Adrenalin 1: 1000 Infectopharm (D)
- as well as generics (A, CH)
Auto-injectors ( solution for injection in pre-filled pen)
- Emerade (D)
- EpiPen (A, CH)
- Fast project (D)
- Jext (D, A, CH, NL, DK, E, I, FIN, N, SLO, S, UK)
- Anapen (D, A, CH) - Lincoln Medical Limited recalled all good-for-use lots on June 5, 2012 due to possible non-delivery of adrenaline.
- InfectoKrupp Inhal (D)
literature
- Klaus Starke : Fundamentals of the pharmacology of the nervous system. In: Wolfgang Forth, Dietrich Henschler , Walter Rummel , Ulrich Föstermann, Klaus Starke: General and special pharmacology and toxicology. 8th, completely revised edition. Urban & Fischer, Munich a. a. 2001, ISBN 3-437-42520-X , pp. 111-146.
- Serafim Guimarães, Daniel Moura: Vascular adrenoceptors: an update. In: Pharmacological Reviews , Volume 53, No. 2, pp. 319-356, PMID 11356987 .
- Otto Westphal , Theodor Wieland , Heinrich Huebschmann: life regulator. Of hormones, vitamins, ferments and other active ingredients. Societäts-Verlag, Frankfurt am Main 1941 (= Frankfurter Bücher. Research and Life. Volume 1), pp. 17–19 ( The adrenal glands ) and 82 f. ( The blood pressure regulator ).
Web links
- Adrenaline - Molecule of the Month (English)
Individual evidence
- ↑ Adrenalin data sheet at AlfaAesar, accessed on December 15, 2010 ( PDF )(JavaScript required) .
- ↑ a b c d Entry on adrenaline in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Entry on (R) -Adrenalin. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ a b c Entry on adrenaline in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ^ William H. Bates: The Use of Extract of Suprarenal Capsule in the Eye. New York Medical Journal, 1896, pp. 647-650 , accessed August 29, 2018 .
- ^ A b c Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Ed. Wiss. Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 166 .
- ↑ John J. Abel: About the part of the adrenal gland that causes blood pressure, the epinephrine. In: Hoppe-Seyler's journal for physiological chemistry . 28, 1899, pp. 318-362, doi: 10.1515 / bchm2.1899.28.3-4.318 .
- ↑ Jeffrey K Aronson: "Where name and image meet". - The argument for "adrenaline". In: British Medical Journal , Volume 320, 2000, pp. 506-509, PMID 10678871 , doi: 10.1136 / bmj.320.7233.506 .
- ↑ Friedrich Stolz : About adrenaline and alkylaminoacetobrenzcatechin . In: Reports of the German Chemical Society . Volume 37, 1904, pp. 4149-4154.
- ↑ Reinhard von den Velden, Die intracardiale injection , Münchner Medizinische Wochenschrift (1919) 66: 274-275.
- ↑ HU Melchert, H. Hoffmeister: Determination of the urinary metabolites hydroxyindole-acetic acid, vanillyl mandelic acid and homovanillic acid by means of lipophilic gel chromatography and gas chromatography. In: Journal of Clinical Chemistry and Clinical Biochemistry. Volume 15 (2). 1977. German. PMID 845547 . Pp. 81-87.
- ↑ a b B. B. Hoffman, RJ Lefkowitz: Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists. In: Louis S. Goodman, Alfred G. Gilman : The pharmacological basis of therapeutics. 10th edition. McGraw-Hill, New York NY et al. a. 2001, ISBN 0-07-135469-7 , pp. 215-268.
- ↑ RW Fuller: Pharmacology of brain epinephrine neurons. In: Annual Review of Pharmacology and Toxicology , Volume 22, 1982, pp. 31-55, PMID 6805416 , doi: 10.1146 / annurev.pa.22.040182.000335 .
- ^ Carsten jewelry , Bernd Engels, Tanja Schirmeister, Reinhold Fink: Chemistry for medical professionals. Pearson studies, Munich a. a. 2008, ISBN 978-3-8273-7286-4 , p. 413.
- ^ Hermann J. Roth , Axel Kleemann : Pharmaceutical Synthesis (= Pharmaceutical Chemistry. Vol. 1). Thieme, Stuttgart a. a. 1982, ISBN 3-13-632901-5 , pp. 14-16.
- ↑ External identifiers or database links for adrenaline hydrogen tartrate: CAS number: 51-42-3, EC number: 200-097-1, ECHA InfoCard: 100.000.089 , GESTIS substance database : 100052 , PubChem : 5815 , ChemSpider : 5610 , Wikidata : Q27255971 .
- ↑ External identifiers or database links for adrenaline hydrochloride : CAS number: 55-31-2, EC number: 200-230-3, ECHA InfoCard: 100.000.210 , GESTIS substance database : 103187 , PubChem : 441411 , ChemSpider : 390147 , DrugBank : DBSALT001484 , Wikidata : Q27107122 .
- ↑ Jerry P. Nolan, Charles D. Deakin, Jasmeet Soar, Bernd W. Böttiger, Gary Smith: European Resuscitation Council guidelines for resuscitation 2005. Section 4. Adult advanced life support. In: Resuscitation. Volume 67, Supplement 1, 2005, pp. S39-S86, PMID 16321716 , doi: 10.1016 / j.resuscitation.2005.10.009 .
- ^ GD Perkins et al .: A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest , In: New England Journal of Medicine, Vol. 379, No. 3, July 18, 2018, doi: 10.1056 / NEJMoa1806842 .
- ↑ Ring J, Beyer K, Biedermann T, Bircher A, Duda D, Fischer et al .: Guideline for acute therapy and management of anaphylaxis. (PDF) In: AWMF. December 1, 2013, p. 6 , accessed August 3, 2019 .
- ^ Red List 2005. Editio-Cantor-Verlag, Aulendorf 2005, ISBN 3-87193-306-6 .
- ↑ Resuscitation Recommendations for Resuscitation . Federal Medical Association, p. 59.
- ↑ Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. (1st edition 1986) 5th edition. Springer, Berlin / Heidelberg / New York a. a. 1999, ISBN 3-540-65024-5 , pp. 44 f. and 76.
- ↑ Recall of the “Anapen” adrenaline autoinjector: Patients should quickly exchange emergency medication due to possible incorrect delivery of the active ingredient ( Memento from December 20, 2015 in the Internet Archive ) Federal Institute for Drugs and Medical Devices, press release from June 4, 2012.