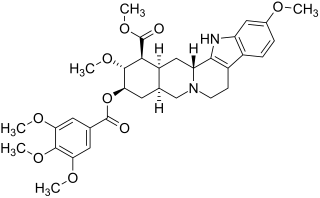
Reserpine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Reserpine | |||||||||||||||||||||
| other names |
(1 S , 2 R , 3 R , 4a S , 13b R , 14a S ) -2,11-dimethoxy-3- (3,4,5-trimethoxybenzoyloxy) 1,2,3,4,4a, 5.7 , 8,13,13b, 14,14a-dodecahydroindolo [2 ', 3': 3,4] pyrido [1,2- b ] isoquinoline-1-carboxylic acid methyl ester ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 33 H 40 N 2 O 9 | |||||||||||||||||||||
| Brief description |
crystalline powder or small crystals, white to pale yellow, slowly darkening under the influence of light |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 608.68 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
264-265 ° C |
|||||||||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Reserpine is a naturally occurring chemical compound that is used as a medicinal substance . It is an indole alkaloid of some plants from the snake root group , which found its way into western medicine mainly via the Rauvolfia serpentina from Indian medicine.
Reserpine is one of those drugs that started the era of modern psychopharmacology . First used in psychiatry as a neuroleptic for schizophrenia , it became particularly important as a remedy for high blood pressure . While it is still of great importance in neurochemical research as a result of research into its mechanism of action , it has largely lost its clinical importance today: after the introduction of much more efficient drugs, reserpine is no longer the drug of choice.
origin
Reserpine is obtained from the roots of climbing plants of the subfamily Rauvolfioideae , primarily from the Indian snake root (Rauvolfia serpentina) , which has a reserpine content of 0.04-0.05%, but also from the Mexican Rauvolfia heterophylla and the Australian bitter bark ( "Iodstrauch"; Tabernaemontana orientalis , also Asternia constricta ). An important alternative source today is the African Rauvolfia vomitoria .
history
In India, the root of Rauvolfia serpentina , also known there as "Sarpagandha" , which also contains yohimbine , has been used for centuries primarily as a sedative , but also as a panacea. At the beginning of the 20th century, it was reported that one of the plant's twenty or more alkaloids had an antihypertensive effect. The first article dealing with the psychiatric use of reserpine was published in 1931 by the Indians Sen and Bose, who reported good treatment results for mental illnesses with violent and manic symptoms. In 1949, a report by Rustom Jal Vakil in the "British Health Journal" made the antihypertensive effects of Rauvolfia known in the western world.
Reserpine was first isolated from Rauvolfia serpentina in 1952 by Emil Schlittler . A short time later he also succeeded in clarifying the chemical structure. Two years later it was recommended and clinically used for the first time by the American psychiatrist Nathan S. Kline for the treatment of psychoses - two years after chlorpromazine , against which it ultimately failed to establish itself as an agent in schizophrenic psychoses, although reserpine was temporarily used after 1952 became one of the most closely related preparations (trade name: Serpasil ) in the treatment of psychiatric illnesses. In the same year, von Freis was the first to report on patients who had become depressed after administration of high doses. In 1958 Robert Burns Woodward published the first total synthesis of reserpin. In the UK, reserpine was withdrawn from the market for several years because of its enormous side effects . However, the well-known psychiatrist Manfred Bleuler wrote in 1969 that the effects of reserpine on the chronically insane were in individual cases more lasting than that of chlorpromazine. In 1958, a combination with orphenadrine (trade name Phasein ) came onto the market in order to reduce the motor side effects . In the late 1970s, reserpine was largely replaced by new, better active ingredients.
The importance of reserpine lies - bearing in mind the fact that it has been largely replaced by better substances in therapy - primarily in its influence on basic research in modern neuropsychopharmacology and neuropsychiatry: Reserpine and its mechanism of action were introduced in the 1950s and 1960s intensively researched, which led to many new insights into biochemical processes, such as the metabolism of biogenic amines, the discovery of regionally reduced concentrations of the neurotransmitter dopamine in the CNS in Parkinson's patients or the preparation for the development of numerous antidepressants based on the observation of reserpine antagonism the MAO inhibitor iproniazid and the tricyclic imipramine .
There are no more reserpine-containing preparations on the market in Germany. The only two preparations that were still among the 3000 most frequently prescribed drugs in 2006 were Briserin N (combination with the thiazide diuretic Clopamid , Novartis ) and Triniton (combination with the antihypertensive drug dihydralazine and the diuretic hydrochlorothiazide , Apogepha ). However, the frequency of prescriptions decreased over the years: Compared to the respective previous year, the medical prescription of Briserin N decreased by 13.1% in 2005 and fell to 29.5 million DDD, in the following year by 14.5% to 25.2 million DDD; at Triniton it fell by 6.9% in 2005 and by a further 16% in 2006 and fell to 6.9 million, then 5.8 million DDD.
The pharmacologists Hartmut Porzig and Stefan Engelhardt suggest that the drug would have fallen into disrepute less, one could previously not such high doses (were added to antihypertensive therapy up to 5 mg per day, for neuroleptic up to 20 mg) with a correspondingly pronounced side effects administered , but significantly lower and at the same time sufficiently effective amounts. The daily doses customary today are between 0.05 mg and 0.25 mg in the treatment of high blood pressure. Dosages of 1 to 2 mg daily have been common in the treatment of schizophrenia.
There have been attempts to develop reserpine derivatives which should be better tolerated than their predecessors. One approach for this, reserpine methonitrate, was presented by the Indians Sreemantula, Boini and Nammi in 2004.
pharmacology
Mechanism of action
The effect of reserpine on the mammalian organism can be divided into a peripheral and a central component. While the former is crucial for lowering blood pressure, the latter is responsible for the effects, because of which reserpine was first used as a neuroleptic, but is now obsolete.
The antihypertensive effect is based on a depletion of the neurotransmitter noradrenaline in the postganglionic sympathetic system . Therefore, although the central sympathetic nerve cells discharge more frequently than before, the stimulus cannot continue to the periphery. As a result of this inhibition of the sympathetic nervous system, in addition to a reduction in the heart rate ( bradycardia ), the desired reduction in blood pressure can also be observed. The antipsychotic effect of reserpine has been linked to an observed decrease in dopamine and serotonin levels in the central nervous system. With a neuroleptic potency of 20–50 CPZi, it is one of the highly potent neuroleptics.
At the cellular level, the mechanism of action of reserpine is based on the "depletion" of biogenic amines such as the messenger substances dopamine, serotonin and noradrenaline. It binds to the non-selective vesicular monoamine transporters in the membranes of the storage vesicles of the presynaptic nerve endings , whereupon the messenger substances can no longer be taken up into the vesicles. In particular, it inhibits the new synthesis of noradrenaline from dopamine, as this takes place inside the vesicles. Outside of this, biogenic amines are broken down by the enzyme monoamine oxidase ("MAO") to aldehydes , ammonia and hydrogen peroxide , which means that the amount of norepinephrine that can be released when aroused is reduced. Excessive dosages lead to irreversible damage to the storage vesicles, which then have to be formed again, which takes a few days to weeks.
Pharmacokinetics
The reserpine taken orally first reaches the stomach and from there into the intestine , where it is quickly absorbed . The absorption rate is increased with a higher pH value of the gastric juice , because the stomach is not only emptied more quickly, but the weakly basic reserpine is also less protonated and in the intestine - whose environment is already comparatively alkaline - is ultimately less electrically charged , which means it can be better absorbed by the intestinal cells. It then reaches the liver for the first time via the portal vein and is then subject to both a first-pass effect and an enterohepatic circulation ; As a result, reserpine, which is only 50–60% available , has two half-lives of 4.5 h and 270 h. Maximum concentrations of the substance in the blood are found around one to two hours after application. 96% of reserpine is bound to plasma proteins , mostly to albumin , but also partly to lipoproteins . Reserpine reaches the peripheral and central nervous tissue via the bloodstream , where it unfolds its medicinally used effect. Since the binding to the neuronal vesicles is irreversible, comparatively very small amounts of the alkaloid are required in order to “de-accumulate” very large amounts of amines from them. The effect of the bound fraction outlasts the general decrease in the concentration in the tissue. Since such small amounts could not be detected with earlier means, it was erroneously assumed that the duration of action of the substance significantly exceeded that of its actual presence at the site of action. This fact coined its designation as hit and run drug . In addition, the lion's share of reserpine is metabolized and then mainly excreted with the faeces (around 60% within four days), partly also in the urine (around 8%).

For elimination , an enzymatic conversion takes place in the intestine and liver, which produces polar and thus more easily excretable substances. The main metabolites - methyl reserpate , trimethoxybenzoic acid (TMBS) and, to a lesser extent, reserpic acid - are produced by esterase- mediated cleavage of the two ester bonds of reserpine. In addition to this hydrolytic way of degradation, catalyzed by microsomal liver enzymes, oxidative demethylation of the middle methoxy group of the TMBS residue takes place. The product can then be more easily hydrolyzed by the organism, with syringic acid being formed instead of TMBS . Compared to the starting substance, the metabolites are pharmacologically largely inactive.
Reserpine methonitrate (RMN) crosses the blood-brain barrier due to the quaternized amine - an amine that is covalently linked to four carbon atoms and thus carries a positive charge - significantly more difficult, which means that significantly less substance is in the CNS occurs than is the case with reserpine, and central nervous side effects (Parkinson's symptoms, sedation) are greatly reduced.
Indication and application
The therapeutic use of reserpine is assessed very differently in the current textbooks against the background of these side effects. While some emphasize the good tolerability of low-dose reserpine and its suitability as a combination partner in the event of inadequate therapy with a single other antihypertensive agent, other authors flatly reject the administration of reserpine, especially Martin Wehling speaks out against it and complains that it is still "in one very popular preparation which is often prescribed due to its low price ” . The drug commission of the German medical profession also carries out the continuous prescription of Briserin N ® and Triniton ® u. a. back to low daily therapy costs, but considers it cheaper and more sensible to initially limit oneself to diuretics. She emphasizes, however, that patients who are already well-adjusted to reserpine combination preparations and who tolerate this medication well have no reason to change anything about them. The last reserpine preparation in Germany ( Briserin N) was withdrawn from the market in 2016.
Reserpine can be used diagnostically to confirm the suspicion of a carcinoid . This reserpine test is a provocation test aimed at the endocrine activity of the tumor : The tumor produces very large amounts of serotonin, which is released from the tumor cells by reserpine and the symptoms typical of carcinoids, including a drastically increased concentration of serotonin. Breakdown product 5-HIES in the urine.
Reserpine is generally contraindicated in patients with a history of depressive episodes, with existing gastric and duodenal ulcers, and with bronchial asthma .
Today, reserpine is usually given orally as a blood pressure lowering agent in long-term therapy , but intramuscular injections were also common in earlier psychiatric use .
Reserpine is recommended in the United States by the Joint National Committee (JNC 8) as an alternative drug for the treatment of high blood pressure. A Cochrane review found reserpine to be as effective as other first-line antihypertensive drugs in lowering blood pressure. The combination of reserpine with a thiazide diuretic is one of the few drug treatments that have been shown to reduce mortality in randomized controlled trials, e.g. For example, the Hypertension Detection and Follow-up Program , the Veterans Administration Cooperative Study Group in Antihypertensive Agents , and the Systolic Hypertension in Elderly Program . In addition, reserpine was included as a secondary antihypertensive option for patients who did not achieve antihypertensive goals in the ALLHAT trial. The daily dose of reserpine in antihypertensive treatment is only 0.05 to 0.25 mg.
Reserpine used to be used in the United States to treat symptoms of dyskinesia in patients with Huntington's disease, but now alternative drugs are preferred.
The use of reserpine as an antipsychotic drug had been almost completely abandoned, but more recently it has been returning as concomitant treatment in combination with other antipsychotics, so that more refractory patients receive dopamine block from the other antipsychotic and dopamine depletion from reserpine. The dosages can be kept low, which leads to better tolerability. Originally, doses from 0.5 mg to 40 mg daily were used to treat psychotic illnesses. Dosages above 3 mg daily, often requiring the use of an anticholinergic drug to treat excessive cholinergic effects in different parts of the body or Parkinson's disease . For concomitant treatment, doses are usually kept at or below 0.25 mg twice daily.
Veterinary medicine
Reserpine is used as a long-acting sedative to calm irritable or troubled horses , and has been used illegally to sedate competition horses, horses for sale, and in other situations where a calmer horse is desired.
Antibacterial effect
Reserpine inhibits the formation of biofilms of Staphylococcus aureus and inhibits the metabolic activity of bacteria in biofilms.
Side effects and interactions
Like most antisympathotonics, reserpine administration can have a number of serious side effects . Since reserpine reduces the availability of catecholamines and thus lowers the sympathetic tone, but acetylcholine - which has its own carrier for entry into the vesicles - leaves undamaged, the activity of the parasympathetic nervous system predominates , and pupil constriction , sagging , swelling of the nasal mucosa ( through serous secretion in this, so-called "reserpine rhinitis"), loss of libido and potency, diarrhea , stomach and duodenal ulcers occur. For the same reasons, in addition to bradycardia as a result of the impaired reflex blood pressure regulation, a position-related drop in blood pressure can occur, which can be so severe, especially when the body is straightened up quickly, that suddenly unconsciousness occurs. The cardiac output , which was reduced by reserpine, normalizes again with chronic administration, since the vascular resistance decreases with the blood pressure .
Reserpine can enter breast milk and damage the fetus. Children whose mothers took reserpine in the last trimester of pregnancy suffer more frequently from drinking and breathing disorders later, newborns can be lethargic and the fetus can develop bradycardia. Reserpine can also cause menstrual cramps .
In the CNS, the lack of dopamine and serotonin is particularly evident, which can lead to extrapyramidal motor disorders, parkinsonism , sedation and depressive moods and even suicidality .
- Paul Willner points out , however, that the widespread belief that reserpine causes depression is based on a number of reports from the 1950s of Goodwin et al. would have been misdiagnosed. They had re-evaluated the data on which the reports at that time were based and came to the conclusion that patients treated with reserpine tended to show pseudo- depression , which is characterized by psychomotor slowdown, fatigue and anhedonia , but not cognitive symptoms of depression such as Feeling hopeless or guilty . According to this, only 5-9% of the patients would have shown symptoms of primary depression, and these would also have had a history of affective disorders .
In the event of a toxic overdose , the heartbeat rate, blood pressure and body temperature drop (which may be preceded by an increase in blood pressure and heart rate), the mucous membranes swell and drowsiness occurs , and cramps may also occur .
Prior administration of tricyclic or tetracyclic antidepressants leads to an effect known as reserpine reversal : tricyclic and tetracyclic drugs prevent transmitters already released into the synaptic gap from being reabsorbed into the presynaptic nerve endings, which bypasses not only the reserpine effect, but also the motor excitement is even increased. After pretreatment with reserpine, indirect sympathomimetics do not work, while the effect of direct sympathomimetics and inhibitors of monoamine oxidase is increased by reserpine. It increases the blood sugar level lowering effect of antidiabetic drugs , whereas the antiparkinsonian effect of bromocriptine and levodopa can be disrupted. If narcotics , opioids or alcohol are taken at the same time , the sedative effects of the substances are mutually reinforcing ; The hypotensive effect is enhanced in combination with vasodilators and thiazide diuretics . The latter is used therapeutically: through the fixed combination of reserpine with the diuretics, which have a completely different mechanism of action, but also lower blood pressure, significantly lower doses are sufficient, which is also associated with a significant reduction in side effects. The neuroleptic effect is intensified without influencing the blood pressure effect when phenothiazines are taken at the same time . In the case of simultaneous intake of cardiac glycosides , cardiac arrhythmias may occur due to the intensification of the effect of the latter .
chemistry
Reserpine is an indole alkaloid of the epialloyohimbane series, which means that its basic structure corresponds to that of yohimbine , but rings C and D as well as rings D and E are cis-linked . This pentacyclic backbone is that of reserpic acid . It is twofold esterified : the acid group at C-16 is methylated , the hydroxy group of reserp acid at C-18 is esterified with 3,4,5-trimethoxybenzoic acid.
Both esters are important for the pharmacological activity of reserpine. The ester cleavages that take place in vivo as part of the metabolism can also be carried out in vitro . Reserp acid methyl ester can be selectively obtained by hydrolysis under mild conditions.
Reserpine is a weak base , wherein the nitrogen atom of N-4 with a pK s value of 6.6 (at 25 ° C) having the highest basicity, accordingly, there occurs the intake of protons . With an octanol-water distribution coefficient (log K OW ) of 3.3, it is a clearly lipophilic substance. Accordingly, it is not dissolved in water, but optimally dissolved in chloroform , but this solution of the labile alkaloid is only of limited durability, especially in daylight, while the solution in ethanol is more difficult to take place, but is the most favorable of the organic solvents in terms of stability.
The fine, crystalline powder, as reserpine, ranks between white, yellowish and a pale yellow-brown in color, but gradually turns darker under the influence of light.
Reserpine has a specific angle of rotation of −116 ° to −128 ° at 20 ° C in sodium light.
Analytics
Various color reactions can be carried out with reserpine. In an acetic acid solution, hydrogen is split off with sodium nitrite , resulting in 3,4-dehydroreserpine. This is a yellow-green fluorescent anhydronium compound, which has a long-wave absorption maximum of 388 nm. Reserpine itself absorbs maximally at 296 nm.
biosynthesis
The biosynthesis of reserpine starts - as well as all other Epiyohimban alkaloids - at Strictosidine . This is created by Mannich-like condensation from tryptamine and secologanin .
Strictosidine is converted to 3-epi-dehydrocorynanthine aldehyde. For this it is first epimerized at C-3 , the β-D-glucose is hydrolytically split off, the later ring D is closed and the later ring E is opened. The order in which these reactions take place is still unknown.
Then 3-epi-dehydrocorynantheinaldehyde is converted into reserp acid methyl ester in three reaction steps:
First, the double bond at N-4 is stereospecifically hydrogenated, so that 3-epi-corynanthine aldehyde is present. Then the later ring E between C-17 and C-18 is closed and the double bond formed between C-19 and C-20 is hydrogenated. Finally, after hydroxylation at C-18 and adding two CH 3 O groups, methyl reserpate is formed.
Finally, methyl reserpate is esterified to reserpine with 3,4,5-trimethoxybenzoyl- CoA on the hydroxyl group at C-18.
Total synthesis
Although reserpine is mainly isolated from Rauvolfia species, fully synthetic production is possible. In a synthesis involving 16 reaction steps, Woodward succeeded in 1958 in the first (constitutional) production of reserpine. In 1958, the total synthesis of reserpine was a milestone in organic chemistry . Due to its complex structure, it has become a classic and the target of numerous total syntheses. In 1989 Gilbert Stork established a way of stereospecific total synthesis . Several alternative approaches have been developed since the initial synthesis.
Woodward's total synthesis begins with the construction of ring E, which contains five of the six stereocenters of the reserpine molecule. The starting materials are 1,4-benzoquinone and methyl penta-2,4-dienoate. A Diels-Alder reaction occurs between the quinone and the diene , the product of which already contains three of the five required stereocenters.
The reduction of the resulting endo adduct with aluminum triisopropoxide is controlled via the cis linkage of the two resulting rings in such a way that, above all, the β-alcohol shown is formed. Since one of the two hydroxyl groups is in close proximity to the methyl carboxylate, a lactone is formed .
The reactivity of the two double bonds in the rings is different to electrophiles : When the C 11 H 12 O 3 is brominated , only the double bond of the later E ring reacts, as it is slightly more electronically rich.
Due to the electrophilic attack of the bromine, the bromonium ion is formed by splitting off a bromide ion. The oxygen of the hydroxyl group opens this three-membered ring through nucleophilic attack on the carbon atom that is sterically more favorable, so that a furan is formed.
In the following, the bromine is substituted by the nucleophilic attack of a methanolate ion , whereby the first of the numerous methyl esters of reserpine is formed: After hydrogen bromide has been eliminated and the α, β-unsaturated lactone has been formed, it is attacked by the methanolate ion. The attack by the methanolation occurs due to the lactone and the ether from the α side, i.e. from below the plane of the drawing, which creates the fifth stereocenter of the E ring.
The second bromination then takes place: N-bromosuccinimide attacks the double bond electrophilically, which could not be broken by Br 2 in the third step . The resulting bromonium ion is opened transdiaxially by nucleophilic attack by water from the α side. Sulfuric acid serves as a catalyst for the reaction .
The product is then oxidized with chromic acid or chromium trioxide .
The next step is a complex reaction that involves removing the bromine atom, breaking two rings, and adding an acetate residue.
The first step here is a radical , reductive debromination. The added zinc first undergoes a single electron transfer into the unoccupied π * orbital of the C – Br bond, whereby the latter is immediately dissolved with the formation of a bromide ion. A secondary, electrophilic carbon radical is formed for a short time, which immediately receives a second electron from zinc and becomes an anion - mesomeric stabilized by the neighboring carbonyl group - which is protonated by the acetic acid . The lactone opening proceeds analogously after previous protonation of the lactone carbonyl group on the oxygen (this lowers the electron density and thus the energetic position of the π * orbital of the C – O bond to be opened).
In the second step, the resulting carboxy group is methylated with diazomethane .
In the third step, the weak base pyridine abstracts the proton from the first step, creating an enolate . In the fourth step, this immediately reacts back to the α-β-unsaturated ketone . With the separated oxygen atom, the acetic anhydride forms an acetyl ester with elimination of an acetate anion at what will later be C-18.
Next, the later D-ring is split open. For this purpose, the double bond is first cis- dihydroxylated with osmium tetroxide , a glycol is formed .
The glycol is then split oxidatively using periodic acid , creating an aldehyde and a ketoaldehyde . The latter is further oxidized by periodic acid via a keto acid and its decarboxylation to the simple carboxy group.
Finally, the carboxy group is methylated with diazomethane, with which the E ring is built up.
The aldehyde is now condensed with the tryptamine 6-methoxytryptamine to form an aldimine , so a double bond is formed between the carbon of the aldehyde group and the nitrogen of the amino group with elimination of water.
The aldimine is then reduced with sodium borohydride . The product cyclizes spontaneously to a lactam . The pyrrole is attacked by reaction with phosphorus oxychloride ( Bischler-Napieralski reaction ), which closes the C ring with the formation of an iminium ion . The iminium ion is then reduced with sodium borohydride.
Due to the spatial structure of the educt, however, the hydride anion attacks from below the molecular level, where it is less hindered. In addition, the thermodynamically more stable product is created in this way, which means that the molecule formed is an epimer of reserpine, but not yet reserpine itself: First, the configuration of the newly created stereocenter at C-3 has to be inverted. For this purpose, two esters are first hydrolytically cleaved with potassium hydroxide , so that a carboxy group is formed at C-16 and a hydroxyl group is formed at C-18 , from which a lactone is built up with the help of dicyclohexylcarbodiimide (DCC). Thereafter, the stability conditions have changed sufficiently that the hydrogen atom at C-3 can be moved to the now more stable β-position with pivalic acid . After the epimerization is complete, the lactone is saponified again.

The last step of the total synthesis according to Woodward consists in the esterification with 3,4,5-trimethoxybenzoyl chloride to racemic (±) -reserpine. The enantiomers are separated by salt formation with camphorsulphonic acid and separation of the diastereomeric camphorsulphonates, as a result of which pure (-) - reserpine could be obtained.
Trade names
Monopreparation for schizophrenia: Sedaraupin (D), Serpasil (D)
Combination with
- Bendroflumethiazide : Tensionorms (F)
- Clopamid and Dihydroergocristin : Normatens (PL)
swell
Main sources
- Forth, Henschler, Rummel: General and special pharmacology and toxicology . Urban & Fischer, Munich / Jena 2001, ISBN 3-437-42520-X , pp. 205-207. [Section # Mechanism of Action ]
- Stitzel: The Biological Fate of Reserpine . In: Pharmacological Reviews (1977), Vol. 28, pp. 179-205. [Section # Pharmacokinetics ]
- Lüllmann, Mohr, Wehling: Pharmacology and Toxicology . Thieme, Stuttgart 2003, ISBN 3-13-368515-5 , pp. 90–91, 545. [Section # Side Effects and Interactions ]
- Rimpler: Biogenic Drugs . Deutscher Apotheker Verlag, Stuttgart 1999, ISBN 3-7692-2413-2 , pp. 309-310. [Section #Biosynthesis ]
- Habermehl, Hammann, Krebs: Natural Products Chemistry . Springer, Berlin 2002, ISBN 3-540-43952-8 , pp. 181-185. [Section #Total Synthesis ]
Individual evidence
- ↑ European Pharmacopoeia Commission (Ed.): EUROPEAN PHARMACOPOE 6TH EDITION . tape 6.0-6.2 , 2008.
- ↑ a b Moffat, Osselton, Widdop, Galichet (eds.): Clarke's Analysis of Drugs and Poisons . Pharmaceutical Press, Bath 2004, ISBN 0-85369-473-7 . Ss. 1531-1532.
- ↑ Reserpine data sheet at AlfaAesar, accessed on February 19, 2010 ( PDF )(JavaScript required) .
- ↑ a b c Reserpine data sheet at Sigma-Aldrich , accessed on May 8, 2017 ( PDF ).
- ↑ Roth, Daunderer, Kormann: Toxic Plants - Plant Poisons . Hüthig Jehle Rehm, 1994, ISBN 3-609-64810-4 , p. 605.
- ↑ a b c Giebelmann, von Meyer: Kulturgeschichtliches zu Hundsgiftgewächsen (2003; PDF; 418 kB).
- ↑ Wink, van Wyk, Wink: Handbook of poisonous and psychoactive plants . Wissenschaftliche Verlagsgesellschaft, Stuttgart 2008, ISBN 3-8047-2425-6 , p. 200.
- ^ Polz: Biosynthesis of Rauwolfia alkaloids . Munich, 1989. (Diss.) Ss. 1-6.
- ↑ Sen, Bose: Rauwolfia serpentina, a new Indian drug for insanity and high blood pressure . In: Indian Med World (1931), Vol. 2, pp. 194-201.
- ↑ a b Kline: Use of Rauwolfia serpentina Benth in neuropsychiatric conditions . In: Annals of the New York Academy of Sciences (1954), Vol. 59, pp. 107-132.
- ↑ Vakil: Antihypertensive affects of Rauwolfia . In: British Health Journal 11, pp. 350-355.
- ↑ a b Langer: Excerpts from a history of psychotropic drugs in the 20th century . In: Langer, Heimann (Ed.): Psychopharmaka - Basics and Therapy . Springer, Vienna 1983, ISBN 3-211-81746-8 . Ss. 25-26.
- ^ Möller, Laux, Deister: Psychiatry and Psychotherapy . Thieme, Stuttgart 2005, ISBN 3-13-128543-5 , p. 492.
- ↑ Urs German: Drug tests at the Psychiatric University Clinic Basel 1953–1980 , pilot study by the University of Bern, March 9, 2017.
- ↑ Wittern: The history of psychotropic drugs before the era of modern psychotropic drugs . In: Langer, Heimann (Ed.): Psychopharmaka - Basics and Therapy . Springer, Vienna 1983, ISBN 3-211-81746-8 , p. 17.
- ↑ Freis: Mental depression in hypertensive patients treated for long periods with large doses of reserpine . In: New England Journal of Medicine , Vol. 251, pp. 1006-1008.
- ↑ a b Bebarta, Dart: Rauwolfia Alkaloids . In: Dart (Ed.): Medical Toxicology . Lippincott Raven, Philadelphia 2004, ISBN 0-7817-2845-2 , p. 712.
- ↑ a b Woodward et al .: The Total Synthesis of Reserpine . In: Tetrahedron (1958), Vol. 2, pp. 1-57.
- ↑ Hans Bangen: History of the drug therapy of schizophrenia. Berlin 1992, page 90 ISBN 3-927408-82-4 .
- ↑ Hans Bangen: History of the drug therapy of schizophrenia. Berlin 1992, page 90 ISBN 3-927408-82-4 .
- ↑ a b c Sreemantula, Boini, Nammi: Reserpine methonitrate, a novel quaternary analogue of reserpine augments urinary excretion of VMA and 5-HIAA without affecting HVA in rats . In: BMC Pharmacology (2004), Vol. 4. Full text .
- ↑ Hans Bangen: History of the drug therapy of schizophrenia. Berlin 1992, ISBN 3-927408-82-4 . P. 90–95: Neuroleptics and psychiatric theorizing
- Jump up : Antihypertensive drugs . In: Schwabe, Paffrath: Drug Ordinance Report 2006 . Springer, Berlin 2006, ISBN 3-540-34369-5 , pp. 418-420. And in: Schwabe, Paffrath: Drug Prescription Report 2007 . Springer, Berlin 2007, ISBN 3-540-72547-4 , pp. 380-381.
- ↑ a b Strong: Pharmacology of noradrenergic and adrenergic systems . In: Forth, Henschler, Rummel: General and special pharmacology and toxicology . Urban & Fischer, Munich / Jena 2001, ISBN 3-437-42520-X , p. 206.
- ↑ a b Kähler: Rauwolfia alkaloids . Boehringer Mannheim, Mannheim, 1970. Ss. 108-113.
- ↑ Porzig, Engelhardt: Pharmaceuticals with an effect on the vegetative nervous system. In Estler, Schmidt (Ed.): Pharmacology and Toxicology . Schattauer, Stuttgart, 2006. p. 144.
- ↑ a b c d Porzig, Häusler: Pharmaceuticals with an effect on the vegetative nervous system . In: Estler (Ed.): Pharmacology and Toxicology . Schattauer, Stuttgart / New York 2000, ISBN 3-7945-1898-5 , p. 96.
- ↑ Hans Bangen: History of the drug therapy of schizophrenia. Berlin 1992, p. 90 ISBN 3-927408-82-4 .
- ↑ Mutschler, Geisslinger, Kreemer, Ruth, Schäfer-Körting: Mutschler drug effects compact . P. 72.
- ↑ Heintze: Pharmaceuticals with an effect on the gastrointestinal tract . In: Estler (Ed.): Pharmacology and Toxicology . Schattauer, Stuttgart / New York 2000, ISBN 3-7945-1898-5 , p. 435.
- ↑ Verspohl: Neuroleptics . In: Ammon (Hrsg.): Drug side effects and interactions . Wissenschaftliche Verlagsgesellschaft, Stuttgart 1991, ISBN 3-8047-1155-3 , p. 255.
- ↑ Chen, Danon: Binding of reserpine to plasma albumin and lipoproteins . In: Biochemical Pharmacology (1979), Vol. 28, pp. 267-271.
- ↑ a b Beyer: Biotransformation der Arzneimittel . Springer, Berlin / Heidelberg / New York 1990, ISBN 3-540-50696-9 , pp. 489-490.
- ^ Cohen: Reserpine . In: Sadée (Ed.): Drug Level Monitoring . John Wiley & Sons, New York / Chichester / Brisbane / Toronto 1980, ISBN 0-471-04881-X , pp. 415-417.
- ↑ Hansel, Sticher: Pharmacognosie - Phytopharmazie . Springer, Heidelberg 2007, ISBN 3-540-26508-2 , pp. 1421-1424.
- ↑ a b c Mutschler, Geisslinger, Kroemer, Ruth, Schäfer-Korting: Mutschler drug effects . Wissenschaftliche Verlagsgesellschaft, Stuttgart 2008, ISBN 978-3-8047-1952-1 , p. 357.
- ^ Wehling: Cardiovascular diseases . In: Wehling (Ed.): Clinical Pharmacology . Thieme, Stuttgart 2005, ISBN 3-13-126821-2 .
- ↑ Drugs Commission of the German Medical Association (Ed.): Drug Ordinances: Recommendations for rational pharmacotherapy . Deutscher Ärzte-Verlag, Cologne 2006, ISBN 3-7691-1201-6 , p. 681.
- ^ Kähler: Rauwolfia alkaloids . Boehringer Mannheim, Mannheim, 1970. Ss. 123-127.
- ^ Roche Lexicon Medicine: Article reserpine . Urban & Fischer, Munich 2006, ISBN 3-437-15156-8 , p. 1585.
- ↑ James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Narva AS , Ortiz E: 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) . In: JAMA . 311, No. 5, February 2014, pp. 507-20. doi : 10.1001 / jama.2013.284427 . PMID 24352797 .
- ↑ Shamon SD, Perez MI: Blood pressure-lowering efficacy of reserpine for primary hypertension . In: Cochrane Database Syst Rev . December 12, 2016, p. CD007655. doi : 10.1002 / 14651858.CD007655.pub3 . PMID 27997978 . PMC 6464022 (free full text).
- ↑ Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow-up Program Cooperative Group . In: JAMA . 242, No. 23, 1979, pp. 2562-71. doi : 10.1001 / jama.242.23.2562 . PMID 490882 . full text at OVID
- ^ Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg . In: JAMA . 202, No. 11, 1967, pp. 1028-34. doi : 10.1001 / jama.202.11.1028 . PMID 4862069 .
- ^ Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group . In: JAMA . 265, No. 24, 1991, pp. 3255-64. doi : 10.1001 / jama.265.24.3255 . PMID 2046107 .
- ↑ ((ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group)): Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Archived from the original on November 26, 2009. In: JAMA . 288, No. 23, December 18, 2002, pp. 2981-97. doi : 10.1001 / jama.288.23.2981 . PMID 12479763 .
- ↑ Shen, Howard: Illustrated Pharmacology Memory Cards: PharMnemonics . Minireview, 2008, ISBN 978-1-59541-101-3 , p. 11.
- ↑ Bhomraj Thanvi, Nelson Lo, and Tom Robinson: Levodopa ‐ induced dyskinesia in Parkinson's disease: clinical features, pathogenesis, prevention and treatment. Postgrad Med J. 2007 Jun; 83 (980): 384-388. PMC 2600052 (free full text).
- ^ Forney B. Reserpine for veterinary use. Available at http://www.wedgewoodpetrx.com/learning-center/professional-monographs/reserpine-for-veterinary-use.html .
- ↑ Parai D, Banerjee M, Dey P, Mukherjee SK: Reserpine attenuates biofilm formation and virulence of Staphylococcus aureus . In: Microb. Pathog. . 138, October 2019, p. 103790. doi : 10.1016 / j.micpath.2019.103790 . PMID 31605761 .
- ^ Bopp, Herbst: Handbook Medicines . Stiftung Warentest , 2010, ISBN 978-3-86851-119-2 .
- ↑ Willner: Dopaminergic Mechanisms in Depression and Mania . In: Psychopharmacology: The Fourth Generation of Progress; Raven Press, Ltd., New York, p. 925.
- ↑ Goodwin, Ebert, Bunney: Mental effects of reserpine in man: a review . In: Shader: Psychiatric complications of medical drugs . New York, Raven Press, 1972, pp. 73-101.
- ↑ Mutschler, Geisslinger, Kroemer, Ruth, Schäfer-Korting: Mutschler drug effects . Wissenschaftliche Verlagsgesellschaft, Stuttgart 2008, ISBN 978-3-8047-1952-1 , p. 1085.
- ↑ Kretzschmar, Stille: Psychotropic drugs . In: Estler (Ed.): Pharmacology and Toxicology . Schattauer, Stuttgart / New York 2000, ISBN 3-7945-1898-5 , p. 237.
- ↑ Verspohl: Neuroleptics . In: Ammon (Hrsg.): Drug side effects and interactions . Wissenschaftliche Verlagsgesellschaft, Stuttgart 1991, ISBN 3-8047-1155-3 , pp. 279-280. And: In short: antihypertensive substances . In: ibid. Ss. 671-672.
- ↑ a b c Eger, Troschütz, Roth: drug analysis . Deutscher Apotheker Verlag, Stuttgart 1999, ISBN 3-7692-2595-3 , pp. 527-531.
- ↑ Auterhoff , Knabe, Höltje: Textbook of Pharmaceutical Chemistry . Wissenschaftliche Verlagsgesellschaft, Stuttgart 1994, ISBN 3-8047-1356-4 .
- ↑ a b Chen, Huan: Reserpine: A challenge for total synthesis of natural products . In: Chemical Reviews, Vol. 105, pp. 4671-4706; doi: 10.1021 / cr050521a .
- ↑ Stork: The Stereospecific Synthesis of Reserpine . In: Pure Applied Chemistry (1989), Vol. 61, pp. 439-442. See also: Org. Chem. Highlights
- ↑ Joelle Gauchet: A Comparative Analysis ot the Syntheses of Reserpine ( Memento of July 14, 2010 in the Internet Archive ), 1992 (synthesis variants in a schematic comparison / English, pdf; 665 kB)
- ↑ References to some of the original works ( Memento of March 31, 2010 in the Internet Archive ) (there from No. 21; PDF; 86 kB)












