Opioids
Opioids (from ancient Greek ὄπιον ópion [ ˈɔpiɔn ], German 'poppy seed juice, opium' and Middle Greek εἶδος eidos [ ˈiðɔs ], German 'shape' , together "similar to opium") is a collective term for a chemically heterogeneous (non-uniform) group of natural and synthetic as well as semi-synthetic substances that have morphine-like properties and are effective at opioid receptors . The term opiate , on the other hand, only stands for the substances naturally occurring in opium with this effect, which are chemically alkaloids and are obtained from the opium poppy (Papaver somniferum) .
A distinction is made between the body's own ( endogenous ) opioids, which play a role in suppressing pain in the context of the stress response, from therapeutically or improperly administered ( exogenous ) opioids.
The spectrum of activity of opioids is complex and very diverse. The most important effect is a strong pain relief ( analgesia ) with low cardiovascular side effects, which makes opioids indispensable and widely used drugs in pain therapy , anesthesia and other areas of application. Sedation and the euphoric effect are among the many other effects ; the most important side effects are decreased vigilance and respiratory depression , v. a. in the event of overdose , as well as constipation (constipation) and development of dependence .
Mode of action
The group of opioids is a chemically heterogeneous subgroup of analgesics . Opioids act as ligands (binding partners) at the orthosteric binding site of the receptors named after them . These opioid receptors are found on the surface of nerve cells and other cells throughout the body; they are most commonly found in the brain at the floor of the fourth ventricle , in other brain regions, and in the spinal cord . They can also be found in the periphery , including in the intestine .
Opioids develop their analgesic effects primarily in the central nervous system . An example of an opioid with only peripheral effects is loperamide , a diarrhea remedy that does not normally cross the blood-brain barrier . It causes the intestinal peristalsis to slow down .
The body's own opioids are endogenous peptides ( enkephalins and endorphins ) that are important in the stress response .
Receptors
Several different types and subtypes of opioid receptors are distinguished:
| Type | localization | effect |
|---|---|---|
| μ 1 and μ 2 | brain | Analgesia , cardiovascular effects |
| μ 2 (MOR or OP 3 ) | spinal , supraspinal | Analgesia , respiratory depression , euphoria , gastrointestinal effects, addiction |
| μ | peripheral | Analgesia, gastrointestinal effects, itching |
| κ (Kappa) (KOR or OP 2 ) | Brain, spinal | Analgesia, sedation , dysphoria |
| δ (Delta) (DOR or OP 1 ) | Brain, spinal, peripheral | Gastrointestinal effect, modulating effect |
| previously unidentified receptor | Miosis , nausea , vomiting |
The σ-receptor is no longer counted among the opioid receptors, because even if some opioids have an agonistic effect on them, neither endogenous opioids bind to this receptor, nor can the effect of selective σ1 ligands be blocked by naloxone / naltrexone.
Agonists and antagonists
Depending on the group, opioids bind differently to the various receptors, whereby they can have an activating ( agonist ) or inhibitory ( antagonist ) effect, creating a complex pattern of action ( multiple receptor theory ). There are four groups:
Pure agonists
Pure agonists have an exclusively activating effect with a high affinity (binding strength) and high intrinsic activity (potency) for μ-receptors as well as a lower affinity for κ-receptors. Meptazinol binds selectively to the μ 1 receptor and thus reduces the μ 2 -mediated respiratory depression if the analgesic effect persists. The effect of these substances can be completely neutralized by antagonists. The combination of pure agonists with mixed agonist-antagonists is not advisable, since this weakens the effect. Most of the opioid drugs used in medicine are pure agonists, examples are tramadol , pethidine , codeine , piritramide , morphine , levomethadone , diethylthiambutene , ketobemidone and the strong analgesics fentanyl , alfentanil , remifentanil and sufentanil .
Mixed agonist-antagonists
Mixed agonist-antagonists offer a complex pattern of action. At μ-receptors they are ligands with a high affinity, but very weak intrinsic activity, so that an antagonistic effect results. In contrast, affinity and intrinsic activity are high at κ receptors (κ agonists). They also have an agonistic effect on δ receptors. In contrast to the pure agonists, there is no further increase in the effects ( ceiling effect ) with increasing dosage . Substances in this group are pentazocine , butorphanol , N-naphthoyl-6β-naltrexamine and nalbuphine , although the pharmacological importance has declined significantly due to the tendency to dysphoria , hallucinations , disorientation and circulatory stimulation (σ-agonists).
Partial agonists
The only pharmacologically relevant substance is buprenorphine , which has a very high affinity at μ-receptors, about 30 times more activity and longer analgesic duration (6 to 8 hours) than morphine. It is also subject to a ceiling effect, although this has no therapeutic relevance, but only lowers the risk of respiratory depression. Buprenorphine has the longest duration of action of any opioid.
Pure antagonists
Pure antagonists act as competitive antagonists on all receptor types, but with different affinities. They are mostly used to neutralize (antagonize) agonistic opioid effects ( termination of anesthesia , antidote in case of intoxication , withdrawal treatment). The active ingredients used are naloxone and naltrexone .
Other representatives
Further examples of opioids are acetorphine , acetyl-α-methylfentanyl , acetyldihydrocodeine , allylprodine , etonitazene , levomethorphan , lofentanil , MDAN-21 , PZM21 and nicomorphine .
Natural opioids and opiates
Endogenous opioids
The endogenous opioids are endogenous peptides that are released as part of the stress response and serve to suppress acute pain and hunger, but also interact with the sex hormones and are involved in the development of euphoria and the regulation of gastrointestinal functions, breathing, thermoregulation and immune reactions. They are released in the event of injuries, but also through emotional stimuli and UV light. Their secretion is also changed in the case of obesity, mental disorders, but also opioid administration. A precise understanding of these complex functions and regulatory processes is still lacking.
The endogenous opioids can be divided into three groups. The precursor peptide of the endorphins is the pro-opiomelanocortine ( POMC ), from which the endorphins α, β and γ arise . From the group of enkephalins are the variants Met-Enkephalin, Leu-Enkephalin and Met-Arg-Phe-Enkephalin, which differ in the N-terminal amino acids. The dynorphins are divided into dynorphins A and B as well as α- and β-neoendorphin.
The endogenous opioids (neuropeptides) are produced in mammals in the hypothalamus and pituitary gland and differ in distribution and receptor affinity.
Opiates / opium alkaloids
Natural substances found in opium are called opiates . The opium obtained from the opium poppy ( Papaver somniferum ) consists of around 25% of these alkaloids. The most important substances are morphine (10%), codeine (0.5%) and thebaine (0.2%) from the phenantrane group as well as the isoquinoline derivative noscapine (6%), papaverine (0.8 to 1%) and narceine (0.3%) which are benzylisoquinolines .
Kratom alkaloids
The leaves of the kratom tree ( Mitragyna speciosa ) contain the opioid alkaloids mitragynine and 7-hydroxymitragynine .
These alkaloids are also found in lower concentrations in other representatives of the genus Mitragyna.
Akuamma
The seeds of the African Akuamma tree ( Picralima nitida ) contain the opioid Akuammin, which is structurally similar to the kratom alkaloids.
Key data on common opioids
| Surname | relative potency | minimal duration of action | classification | Remarks | Classification under narcotics law (Germany) |
|---|---|---|---|---|---|
| [[ N - [(3 R , 4 S ) -1 - [(2 S ) -2-Hydroxy-2-phenyl-ethyl] -3-carboethoxy-4-piperidyl] - N -phenyl-propanamide]] | 30000 | ? | Agonist | Agonist | Strongest known analgesic, not available for a prescription (see Ohmefentanyl ) |
| [[ N - [(3 R , 4 S ) -1 - [(2 S ) -2-hydroxy- (2- {4-fluoro} phenyl-ethyl)] - 3-methyl-4-piperidyl] - N -phenyl-propanamide]] | 18000 | ? | Agonist | Agonist | not prescribable (see ohmefentanyl ) |
| Carfentanyl | 10,000 | ? | Agonist | Agonist, for stunning large animals | Most powerful veterinary analgesic, BtMG |
| Dihydroetorphine | <10000 | ? | Agonist | Agonist, used in China | |
| Ohmefentanyl | 6300 | ? | Agonist | Agonist | |
| Etorphine | 2000 | ? | Agonist | Agonist | |
| Sufentanil | ≈1000 | 30 min | Agonist | Most powerful human analgesic | prescription narcotic drug (BtM) |
| Remifentanil | ≈100-200 | 8-10 min | Agonist | Very short half-life , therefore very easy to control. Mainly used in the context of TIVA . | prescription-ready BtM |
| Fentanyl | 120 | 30 min | Agonist | transdermally applicable | prescription-ready BtM |
| Alfentanil | 30-40 | 10 min | Agonist | transdermally applicable | prescription-ready BtM |
| Buprenorphine | ≈30 | 6–8 h | partial agonist | Has an analgesic effect when given alone, antagonistic in combination with agonists; Ceiling effect | prescription-ready BtM |
| Hydromorphone | 7.5 | 3–5 h (without delay) | Agonist | The sedative effect is significantly less than that of morphine | prescription-ready BtM |
| Levomethadone | 4th | 5-7 h | Agonist | Cumulation and strong increase in half-life with daily administration. | prescription-ready BtM |
| Diacetylmorphine (Heroin) / Diamorphine (DAM) | 2.5 | 3–4 h (metabolites) | Agonist | very high potential for dependency | exclusively for the production of preparations for medicinal purposes marketable narcotics, exclusively for replacement therapy prescription narcotics enabled |
| Methadone | 2 | 5-7 h | Agonist | see levomethadone | prescription-ready BtM |
| Oxycodone | 1.5-2 | 3.5–7 h (without delay) | Agonist | prescription-ready BtM | |
| Hydrocodone | 1.5 | 4-8 h | Agonist | prescription-ready BtM | |
| Morphine | 1 | 2-4 h | Agonist | Reference substance for opioids, actually an opiate | prescription-ready BtM |
| Piritramide | 0.7 | 4-6 h | Agonist | prescription-ready BtM | |
| Nalbuphine | 0.5-0.7 | 3-6 h | mixed agonist-antagonist | not regulated by narcotics law | |
| Tapentadol | 0.3-0.5 | 4 h (for non-retarded tapentadol) | Agonist at the μ-opioid receptor, selective norepinephrine reuptake inhibitor | prescription-ready BtM | |
| Pentazocine | 0.3 | 2-4 h | mixed agonist-antagonist | prescription-ready BtM | |
| Dihydrocodeine | 0.2 | 3-4 h | Agonist | Prescription BtM, with the exception of preparations below a specified dose limit | |
| Pethidine | 0.1-0.2 | 2-4 h | Agonist | prescription-ready BtM | |
| Tilidine | 0.1-0.2 | 3-4 h | Agonist | trans -Tilidine: a prescription narcotic drug, with the exception of preparations below a specified dose limit and in combination with naloxone; cis -tilidine: marketable, but not prescribable. | |
| Codeine | 0.1 | 4 h | Agonist | an opiate | Prescription BtM, with the exception of preparations below a specified dose limit |
| Meptazinol | 0.1 | 3-4 h | my2 agonist | not regulated by narcotics law | |
| Tramadol | 0.1 | 4 h | Agonist | not regulated by narcotics law | |
| Naloxone | against 0 | 1-4 h | pure antagonist | Duration of action only applies to intravenous administration | not regulated by narcotics law |
| Naltrexone | against 0 | up to 24 h | pure antagonist | oral administration | not regulated by narcotics law |
| Diprenorphine | against 0 | ? H | pure antagonist | strongest opioid antagonist and therefore also effective with highly potent opioids such as buprenorphine or etorphine | not regulated by narcotics law |
| Loperamide | against 0 | effective only on peripheral opioid receptors, treatment of diarrhea | not regulated by narcotics law | ||
| Apomorphine | against 0 | Emetic , on dopamine receptors in the area postrema | not regulated by narcotics law |
Effects
analgesia
The pain relief is the desired effect in the use of opioid analgesics. It is mainly mediated via the μ receptors, especially μ 1 , but also via κ.
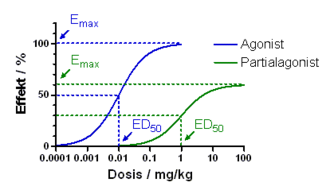
The potency of the individual substances is called the analgesic potency , which is specified relative to morphine, the value of which is set as 1. The higher the analgesic potency, the lower the dose of a drug required to produce comparable analgesia. Pharmacodynamically , the potency can be given with the effective dose ED 50 .
The maximum achievable analgesia means that with low-potency opioids the analgesic effect only increases up to a certain dose increase, in contrast to high-potency substances, a further increase does not lead to stronger analgesia, but an increase in undesirable effects. Pharmacodynamically, the maximum achievable analgesia is a measure of the intrinsic activity of an active ingredient. These values are also genetically determined to a large extent.
Opioid analgesics are part of the WHO level scheme for the treatment of chronic pain. In the second stage, this provides for the administration of a low-potency opioid in addition to a non-opioid analgesic (first stage); in the third stage, the use of a highly potent substance. In addition, opioid analgesics are used therapeutically in many other areas of medicine, for example for induction of anesthesia in anesthesia and many acute pain-associated diseases and injuries in emergency and intensive medicine that cannot be controlled with non-opioid painkillers . Opioids are often packaged in safety blisters rather than regular push-through blisters. This serves to prevent accidental ingestion or as a child safety device .
For long-term therapy of chronic, non-tumor-related pain, opioids are only effective to a limited extent and are only indicated when taking into account the actual pain relief (recorded using the visual analogue scale ), the possible side effects even of the lower-potency pain medication and with the additional use of additional pain-relieving measures (see also pain therapy ).
Respiratory depression
The unwanted respiratory depression (as a reduced responsiveness of the respiratory center to respiratory urges ) is triggered by a reduced CO 2 sensitivity of the respiratory center via μ 2 receptors. It is directly proportional to the analgesic potency of the opioid. Indirectly, it also increases intracranial pressure ( intracranial pressure ) through vasodilation (widening of the vessels) . Hypoventilation (decreased breathing) with only a few breaths per minute occurs as a mild form . It is typical for this that the person concerned complies with a request to actively breathe (so-called command breathing ). Breathing comes to a standstill at higher doses . Respiratory depression can be reversed by using the antagonist naloxone.
With pain-oriented administration of opioids, there is usually no clinically relevant respiratory depression as long as the opioid administration is based on the degree of pain reduction and an overdose is avoided. Pain is an opioid antagonist with respect to respiratory depression.
Respiratory distress therapy in palliative medicine
Opioids can be administered in the treatment of dyspnea as a consequence of advanced diseases such as COPD at least in the terminal stage of the disease, provided that the aim is to alleviate symptoms and not to accelerate the dying process. In addition, opioids are also helpful in relieving breathlessness in COPD patients outside of the terminal stage, which cannot be treated otherwise. For use z. B. Morphine by intravenous or subcutaneous injection, another possibility is the administration of nasally administered fentanyl .
Psychotropic Effects
A sedation (tranquilizers) is effected via κ receptors. It is partly desired (anesthesia, sedation in sedation ), partly undesirable (long-term pain therapy). Even with high doses of opioid analgesics, consciousness is not safely switched off, so that opioids are generally combined with inhalative or intravenous hypnotics as part of general anesthesia , in order to avoid wakefulness phenomena ( awareness ).
Opioids also have anti-anxiety and euphoric effects, which are believed to be responsible for the psychological component of opioid addiction . In addition, dysphoria and hallucinations can also be caused via σ-receptors , which plays a role in the mixed agonist-antagonists.
Nausea and vomiting
By stimulating dopamine- dependent receptors in the trigger zone of the area postrema on the floor of the fourth ventricle , the vomiting center in the formatio reticularis is stimulated and thus nausea and vomiting ( emetic effect) is triggered. In the context of anesthesia, this can occur postoperatively ( postoperative nausea and vomiting , PONV ). This effect can be mitigated by antiemetics . In higher doses, however, opioids dampen the vomiting center, so that an antiemetic (nausea-reducing) effect then results.
Apomorphine , which is related to morphine, has a pronounced effect on the dopamine-2 receptors in the area postrema. For this reason it can be used to induce vomiting in some cases of poisoning, but is no longer approved in Germany for this indication (in humans).
Constipation
Spastic constipation of the intestine (constipation) is caused by the stimulation of μ-receptors of the myenteric plexus of the intestinal wall with a constriction of the smooth muscles . They are the most relevant side effect in long-term pain treatment and are only subject to low tolerance development. Lactulose can be given prophylactically .
The constipating effect is desirable when using the morphine derivative loperamide , which is used as an antidiarrheal for the symptomatic therapy of severe diarrhea .
Other effects
The odd sphincter also constricts , which increases the pressure in the bile duct system, which can lead to colic-like pain. In addition, a congestion of secretion in the pancreas and, as a result, pancreatitis is possible.
The same mechanism causes voiding disorders of the urinary bladder with urinary retention .
The dampening of the cough center results in an antitussive (cough-reducing) effect. (This has also been described selectively for the non-morphine-like opium alkaloid and isoquinoline derivative noscapine ). This effect is used in the antitussive codeine and derivatives. However, the rapid injection of highly potent synthetic opioids such as fentanyl during induction of anesthesia can initially lead to a coughing irritation.
Opioids cause central sympathicolysis (reduced activity of the sympathetic nervous system). This, along with an increase in the activity of the vagus nerve and direct vasodilation , leads to a drop in heart rate ( bradycardia ), blood pressure ( hypotension ) and cardiac output . In the usual therapeutic dosage, the impairment of the cardiovascular function is only minor. With restricted circulatory regulation such as volume depletion ( shock ), anesthesia and antihypertensive drugs (antihypertensive drugs), a critical drop in blood pressure is possible. In the therapy of acute myocardial infarction (heart attack) and acute left heart failure (cardiac insufficiency), the circulatory depressant effect is used to relieve the cardiac function and to reduce myocardial oxygen consumption.
A miosis (contraction of the pupil ) is determined by the stimulation of the parasympathetic nucleus Edinger-Westphal in the midbrain and contraction caused thereby triggered the sphincter muscle of the pupil. In the presence of opioid poisoning with oxygen deficiency (see below), mydriasis (dilation of the pupil) can also occur.
Bolus injections of highly potent opioids during anesthesia can trigger muscle rigidity, which mainly affects the thorax (chest) and abdomen ( wooden chest ), which makes mask ventilation difficult when anesthesia is induced. The mechanism is unclear. This undesirable effect is particularly pronounced with alfentanil and remifentanil . In the context of modern combination anesthesia, this phenomenon only plays a subordinate role due to the muscle relaxants used .
Opioids can still cause itching ( pruritus ). This comes about through an overlap of pain- and itching-associated peripheral mediators and receptors.
Opioids are not triggers for malignant hyperthermia and have no toxic (poisonous) effects on the liver or kidneys . They release histamine .
Intoxication
Acute poisoning with opioids can occur in the context of an overdose in the case of addiction, usually with ignorance of the opioid concentration and with suicidal intent, accidentally (unintentionally), iatrogenic (as a result of medical treatment) or in the case of a body-packer syndrome in drug couriers . In addition, when taking benzodiazepines at the same time, the risk of an opioid-associated overdose is up to five times higher than when taking opioids alone.
The typical symptom triad of opioid intoxication consists of respiratory depression, coma that cannot be awakened and miosis with pupils the size of a pinhead. Mydriasis can also occur if the patient is deeply unconscious. Inadequate breathing results in hypoxia (lack of oxygen) with cyanosis , possibly leading to pulmonary edema . Cheyne-Stokes breathing may be observed. Circulatory weakness with bradycardia (slow pulse) and hypotension (drop in blood pressure) also occurs . The muscle tone is reduced, the reflexes are weakened or lost. In the differential diagnosis, clonidine poisoning comes into consideration, the appearance of which can be very similar.
Treatment of opioid poisoning is primarily symptomatic and consists in safeguarding vital functions . The most important measure is to keep the airways free in the event of respiratory failure, if necessary through endotracheal intubation and controlled ventilation with oxygen. The circulatory insufficiency requires shock treatment with volume administration via large-lumen intravenous cannulas . If cramps occur, they are treated with benzodiazepines .
As a specific antidote is naloxone used which acts as an antagonist at all opioid receptors. This is used in repetitive doses until the clinical symptoms have significantly improved (titration antagonization). Caution is advised with addicts, in whom this can trigger an acute withdrawal syndrome. When antagonizing longer-acting opioids, a rebound phenomenon , remorphinization, can occur because of the very short half-life of naloxone .
According to the 2017 World Drug Report, opioids are the most harmful intoxicating drugs associated with illicit drug abuse and are responsible for 70% of all negative health effects worldwide.
Tolerance, dependence and withdrawal
The development of tolerance (habituation) with opioids refers to the weakening of the effect and the shortening of the duration of action with repeated administration, which is compensated for by increasing the dose. It is a pharmacodynamic tolerance that is mainly based on an increased enzyme activity of the intracellular adenylate cyclase .
The development of tolerance is characteristically faster for the analgesic, euphoric and respiratory depressive effects than for the spasmogenic peripheral effects and miosis. In the case of endogenous opioids, the development of tolerance is prevented by the uptake and processing of the receptors in the cell. If the opioid intake is interrupted, withdrawal symptoms occur , which are based on an increased release of norepinephrine . Withdrawal symptoms are a diagnostic criterion for a resulting dependence .
A withdrawal of opioids can in the outpatient or inpatient care or without medical assistance as so-called. "Cold turkey" or performed with "qualified" methods. The severity of the opioid withdrawal syndrome can be assessed using various validated assessment sheets and depends on several factors. A psychosocial care , which is also a contributing factor for a successful withdrawal in and of itself, moreover, this is to follow, since it is a prolonged and continuous abstinence is facilitated. Psychosocial care is also required for people who are in substitution programs . The latter are the recognized therapy for addiction to opioids, especially heroin addiction.
If opioids are used for pain therapy, there is no development of tolerance and dependence (with sufficient and time-constant dosage). A loud desire for increased opioid doses should be seen as a sign of underdosage.
Legal Aspects
The national laws are based on the standard convention on narcotics , formerly the international opium convention .
In Germany the marketability of narcotics is regulated by the Narcotics Act (BtMG), in Switzerland ( Narcotics Act ) and Austria ( Narcotics Act ) corresponding laws apply. Depending on the addiction or abuse potential, opioids are restricted either in all concentrations (see also overview table), from a certain concentration ( codeine , tilidine with naloxone , dextropropoxyphene ) or not at all ( tramadol and nalbuphine ).
See also
literature
- Eckart J, Jaeger K, Möllhoff Th. (Ed.): Kompendium Anästhesiologie , ecomed 2010, ISBN 978-3-609-71361-8 .
- Karow, Lang-Roth: General and special pharmacology and toxicology. 14th edition 2005. Self-published.
- S3 guideline : Long-term use of opioids for non-tumor-related pain (LONTS) , AWMF register number 145/003, status 09/2014
- Jan M. van Ree, Mirjam AFM Gerrits, Louk JMJ Vanderschuren: Opioids, Reward and Addiction: An Encounter of Biology, Psychology, and Medicine. Pharmacological Reviews 51 (2), 1999, pp. 341–396 ( online full text )
- Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. (1st edition 1986) 5th edition. Springer, Berlin / Heidelberg / New York a. a. 1999, ISBN 3-540-65024-5 , pp. 26-35.
- Eberhard Klaschik : Pain therapy and symptom control in palliative medicine. In: Stein Husebø , Eberhard Klaschik (ed.): Palliative medicine. 5th edition, Springer, Heidelberg 2009, ISBN 3-642-01548-4 , pp. 207-313, here: pp. 229-263 ( opioid analgesics ).
Web links
Individual evidence
- ^ Wilhelm Pape , Max Sengebusch (arrangement): Concise dictionary of the Greek language . 3rd edition, 6th impression. Vieweg & Sohn, Braunschweig 1914 ( zeno.org [accessed on September 17, 2019]).
- ^ Wilhelm Pape , Max Sengebusch (arrangement): Concise dictionary of the Greek language . 3rd edition, 6th impression. Vieweg & Sohn, Braunschweig 1914 ( zeno.org [accessed on September 17, 2019]).
- ^ J. Porter, H. Jick: Addiction rare in patients treated with narcotics. In: New England Journal of Medicine , Volume 302, 1980, p. 123.
- ↑ a b Enno Freye: Opioids in medicine. 8th edition. Springer, 2010.
- ^ WR Martin, CG Eades, JA Thompson, RE Huppler, PE Gilbert: The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog . In: J. Pharmacol. Exp. Ther. tape 197 , no. 3 , 1976, p. 517-532 , PMID 945347 .
- ↑ technical information Meptid (R) as of September of 2007.
- ↑ DJ Rowbotham: Endogenous opioids, placebo response, and pain. In: Lancet , Vol. 357, No. 9272, June 16, 2001, pp. 1901-1902. PMID 11425407 .
- ↑ Lüllmann, Mohr: Pharmakologie und Toxikologie , 15th edition 2003. ISBN 3-13-368515-5
- ^ Kratom (Mitragyna speciosa). European Monitoring Center for Drugs and Drug Addiction , accessed October 14, 2017.
- ↑ JR Menzies, SJ Paterson, M. Duwiejua, AD Corbett: Opioid activity of alkaloids extracted from Picralima nitida (fam. Apocynaceae) . In: European Journal of Pharmacology . tape 350 , no. 1 , May 29, 1998, ISSN 0014-2999 , pp. 101-108 , PMID 9683021 .
- ↑ a b Jochen Schulte am Esch, Hans Werner bause, Eberhard Kochs: anesthesia, intensive care, emergency medicine, pain therapy. Thieme, Stuttgart, 3rd edition 2006. ISBN 3-13-119083-3 .
- ↑ a b Karow, Lang-Roth: General and special pharmacology and toxicology. 14th edition 2005. Self-published.
- ^ Frank Detlev, Richling Schneider: Facts. Medicines 2007 . Thieme, Stuttgart 2006, ISBN 3-13-140543-0 .
- ↑ Martin S. Angst, Nicholas G. Phillips et al .: Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. In: Pain. 2012. doi: 10.1016 / j.pain.2012.02.022 .
- ^ H. McQuay: Opioids in pain management. Review. In: The Lancet , Vol. 353, No. 9171, June 26, 1999, pp. 2229-2232. PMID 10393001 .
- ↑ AWMF guideline: Long-term use of opioids for non-tumor-related pain (LONTS) (PDF; 1.3 MB), accessed on December 16, 2015.
- ↑ Husebø, Klaschik: palliative care , Springer Medizin Verlag Heidelberg, 2006, 4th Edition. ISBN 978-3-540-29888-5 .
- ^ AJ Jennings, AN Davies, JPT Higgins et al .: A systematic review of the use of opioids in the management of dyspnoea. In: Thorax , Volume 57, 2002, pp. 939-944.
- ↑ James R. Runo, E. Wesley Ely: Treating dyspnea in a patient with advanced chronic obstructive pulmonary disease - Evidence-Based Case Review. In: West J Med. , Volume 175, No. 3, September 2001, pp. 197-201. PMC 1071542 (free full text).
- ↑ G. Rocker, R. Horton, D. Currow, D. Goodridge, J. Young, S. Booth: Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. In: Thorax , Volume 64, No. 10, October 2009, pp. 910-915. PMID 19786716 .
- ↑ B. Varkey: Opioids for palliation of refractory dyspnea in chronic obstructive pulmonary disease patients. In: Curr Opin Pulm Med. , Volume 16, No. 2, March 2010, pp. 150-154. PMID 20071992 .
- ↑ Shortness of breath in palliative care, Pain Therapy, DGS, edition 2.2011, 27th vol.
- ↑ A. Ikoma et al .: The neurobiology of itch. (Review) In: Nat Rev Neurosci , Volume 7, No. 7, July 2006, pp. 535-547. PMID 16791143 .
- ^ I. Hernandez, M. He, MM Brooks et al .: Exposure-Response Association Between Concurrent Opioid and Benzodiazepine Use and Risk of Opioid-Related Overdose in Medicare Part D Beneficiaries. In: JAMA Network Open , June 22, 2018, 1 (2): e180919. doi: 10.1001 / jamanetworkopen.2018.0919
- ↑ agt: World Drug Report 2017. Retrieved December 5, 2017 .
- ^ World Drug Report. Retrieved December 5, 2017 .
- ↑ E. Freye, L. Latasch: Development of tolerance under opioid administration - molecular mechanisms and clinical significance. In: Anesthesiol. Intensive med. Emergency med. Painful. , Vol. 38, No. 1, January 2003, pp. 14-26. PMID 12522725 .
- ↑ T. Koch et al .: Receptor endocytosis counteracts the development of opioid tolerance. In: Mol Pharmacol . , Volume 67, No. 1, January 2005, pp. 280-287. PMID 15475572 .
- ^ J. Cami, M. Farre: Drug addiction. (Review) In: N. Engl. J. Med. , Vol. 349, No. 10, September 4, 2003, pp. 975-986. PMID 12954747 .