Papaverine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
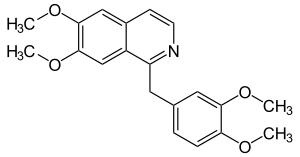
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Papaverine | |||||||||||||||||||||
| other names |
1 - [(3,4-Dimethoxyphenyl) methyl] -6,7-dimethoxyisoquinoline ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 20 H 21 NO 4 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 339.39 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
147-148 ° C |
|||||||||||||||||||||
| pK s value |
6.93 |
|||||||||||||||||||||
| solubility |
very bad in water (35 mg l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Papaverine (from Latin papaver "poppy") is a chemical substance from the group of isoquinoline alkaloids and has a direct antispasmodic effect on the smooth muscles without having an anticholinergic effect at the same time .
Occurrence
Papaverine is the dried milky juice of the opium poppy ( opium ) and related poppy species such. B. the poppy occurring natural substance .
Papaverine is about one percent in raw opium, but as a pure substance does not show its spectrum of activity, since raw opium contains a number of other potent alkaloids whose effects exceed those of papaverine, especially the effect of morphine is in the foreground in opium.
Pharmacological properties
Papaverine is - like its derivative ( derivative ) moxaverine - a cAMP - phosphodiesterase inhibitor . It acts on numerous subtypes of the phosphodiesterase family, but mainly on type 10A. The slackening effect on the vascular muscles leads to vasodilation ( vasodilation ). In higher doses, papaverine can have a central excitatory effect.
use
Papaverine is indicated in cardiac surgery to prevent blood vessel spasms when obtaining arterial grafts, i.e. arteries for a bypass operation. As an antispasmodic for stomach, intestinal, biliary and urinary tract spasms, it has meanwhile been used by other antispasmodics such as B. propiverine , which also have an anticholinergic effect, replaced.
Papaverine is also indicated for the treatment of erectile dysfunction . For this purpose, it is directly into the corpora cavernosa of the penis injected where it leads to an increase in arterial blood flow (so-called. SKAT , d. E. S chwell k örper- A utoinjektions t herapie). The side effects of this method are in part not insignificant. Thus, priapism reported (painful erections without permanent sexual arousal of up to 36 hours) and inflammation of the penis.
The treatment of peripheral and cerebral circulatory disorders with papaverine is controversial.
chemistry
The first total synthesis of papaverine was accomplished by Pictet and Gams in 1909. Starting from veratrol and veratrol-4-carboxaldehyde, papaverine is obtained in eight steps. The structure elucidation succeeded the Austrian chemist Guido Goldschmiedt .
By oxidation with potassium permanganate produced Papaveraldin .
Papaverine hydrochloride is used pharmaceutically . Demelverine also has a similar spasmolytic effect .
Trade names
Paveron N (D)
Androskat (A), Papveron (A), Spasmosol (CH)
Web links
- Horst Josef Koch, Christoph Raschka: Toxicology of the vasodilating alkaloid papaverine ( Memento from June 26, 2003 in the Internet Archive ), In: Die Österreichische Apothekerzeitung, edition 8/2003.
Individual evidence
- ↑ a b c Entry on papaverine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ Louis Fieser and Mary Fieser , Organic Chemistry , 2nd Edition, Verlag Chemie 1982, ISBN 3-527-25075-1 .
- ↑ a b Entry for CAS no. 58-74-2 in the GESTIS substance database of the IFA , accessed on April 16, 2011(JavaScript required) .
- ↑ Entry on Papaverine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b J. Schmidt: About the research of the constitution and the attempts to synthesize important plant alkaloids , p. 111, 1st edition, Bibliobazaar, ISBN 1-103-18707-4 .
- ↑ Siuciak JA, Chapin DS, Harms JF et al. Inhibition of the striatum-enriched phosphodiesterase PDE10A: a novel approach to the treatment of psychosis. Neuropharmacology . 2006; 51 : 386-96. PMID 16780899 .
- ↑ R. Mann Hold: Inhibition of calmodulin dependent c-AMP phosphodiesterase by moxaverine and papaverine . In: Arzneimittelforschung , Vol. 38/12, 1988, pp. 1806-1808. PMID 2854468 .
- ↑ ABDA database (as of August 5, 2008) of DIMDI.
- ↑ K. Hardtke et al. (Ed.): Commentary on the European Pharmacopoeia Ph. Eur. 5.0, papaverine hydrochloride. Loose-leaf collection, 22nd delivery 2005, Wissenschaftliche Verlagsgesellschaft Stuttgart.
- ↑ SV Bhat, BA Nagasampagi, M. Sivakumar: Chemistry of Natural Products , 1st Edition, Springer Verlag, Berlin, 2005, ISBN 3-540-40669-7 , S. 255th
- ↑ J. Schmidt: About the research of the constitution and the attempts to synthesize important plant alkaloids , p. 114, 1st edition, Bibliobazaar, ISBN 1-103-18707-4 .
