Lofentanil
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
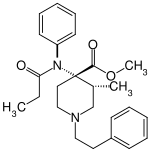
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Lofentanil | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 25 H 32 N 2 O 3 | |||||||||||||||
| Brief description |
white, odorless powder (lofentanil hydrogen oxalate) |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 408.53 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
177 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lofentanil is a synthetically produced chemical compound from the group of opioids that is highly effective as a pain reliever . It was developed in 1975 by the research laboratories at Janssen Pharmaceutica and is originally derived from fentanyl . The analgesic effect is about 6000 times that of morphine .
Background and development
Studies of the connections between chemical structure and biological effect showed in the early 1970s that the introduction of a 3-methyl group in fentanyl increases the potency by up to 19-fold ((+) - cis -3-methylfentanyl). Apart from the considerable increase in potency, other pharmacological properties were less changed. Only the duration of action of 3-methylfentanyl is significantly longer and lies between fentanyl and morphine. The therapeutic index (safety margin) of 3-methylfentanyl is also higher than that of fentanyl (1662: 255). The results were published in 1973 and 1974.
The findings were then transferred to carfentanil: the introduction of a 3-methyl group into the carfentanil molecule leads to four isomers (two optically active cis and two trans isomers each ), which differ significantly in their activity and were separately subjected to a pharmacological screening . (-) - cis -3-methylcarfentanil was identified as the most potent and most effective isomer , which initially ran under the number R 34995 and was given the name LOFENTANIL. This substance was first mentioned in the scientific literature together with carfentanil and sufentanil in 1976.
Pharmacological properties
Lofentanil is a highly lipophilic (more lipophilic than carfentanil), high affinity, highly potent and extremely long-acting opioid. In the case of the fentanyl molecule, the introduction of a 3-methyl group increased the potency by up to 19-fold ((+) - cis -3-methylfentanyl), in the case of carfentanil the potency is slightly reduced by the analogous substitution.
power
For racemic (±) - cis -3-methylcarfentanil (R 32792) the 0.5-0.7-fold potency of carfentanil and the 4600-5600-fold potency of morphine are given. Lofentanil (R 34995) is about 1.2 times more potent than racemic (±) - cis -3-methylcarfentanil (R32792) and 5500-6500 times more potent than morphine. The threshold dose of lofentanil in humans at which the first effects typical of opioids such as nausea, vomiting, sedation and mild analgesia occur is in the range of 250–750 nanograms after intramuscular injection. It is a little more lipophilic than carfentanil, and it works a little faster.
Duration of action
The duration of action is influenced by the 3-methyl group much more than the potency. Lofentanil and R 32792 are among the opioids with the longest duration of action, which can increase dramatically to 72 h and longer with increasing dose.
Duration of action at 4 × ED 50 : R 32792 > 8 h, morphine = 2.61 h, carfentanil = 1.55 h.
The long duration of action results from the binding properties and the great metabolic stability. The lofentanil-µ-receptor complex shows a very high stability and dissociates only very slowly in the presence of large amounts of dextromoramide . The µ-binding affinity is not reduced by sodium ions (uncommon for opioid agonists; cf. “sodium index” of opioid agonists, partial agonists and antagonists).
Toxicity and therapeutic index (safety margin)
The toxicity (effect on the respiratory center) also increases sharply. LD 50 values: morphine = 223 mg / kg, carfentanil = 3.4 mg / kg, R 32792 = 0.2 mg / kg, lofentanil = 0.066 mg / kg.
The following therapeutic indices were calculated from animal experiments: morphine = 69; Lofentanil (R 34995) = 112; Fentanyl = 282; R 32792 ("raz.-Lofentanil") = 285;
(+) - cis -3-methylfentanyl = 1662; Carfentanil = 10,000.
Receptor selectivity
In contrast to carfentanil (a selective µ-agonist), lofentanil is an unselective opioid receptor agonist, which binds with high affinity to the µ-receptor, but also significantly to δ- and κ-receptors.
Table: Binding affinity (K i [nM]) of lofentanil to opioid receptors in comparison with other fentanyl derivatives
| substance | µ (DAGO) | δ (DPDPE) | κ (U 69.593) |
|---|---|---|---|
| Lofentanil | 0.023 | 0.24 | 0.60 |
| Carfentanil | 0.024 | 3.3 | 43 |
| Fentanyl | 1.2 | 180 | 290 |
| R 30490 | 0.09 | 23 | 63 |
R 30490 = 4-methoxymethylfentanyl (sufentanil analogue)
Clinical studies with lofentanil
In 1983, a first clinical study (120 patients) compared lofentanil (250, 500 and 750 nanograms im) with piritramide (7.5 and 15 mg im) and placebo . In the time window of 4–6 hours after application, 15 milligrams of piritramide were superior to 750 nanograms of lofentanil in terms of analgesic effects and also showed a longer duration of action. The side effects were comparable in both groups (nausea, vomiting, sedation). In 1985, lofentanil with buprenorphine was clinically tested for postoperative pain with extradural application (L3-L4). 5 µg lofentanil showed a longer duration of action (up to 72 h) and a stronger effect than 300 µg buprenorphine. Some patients became sleepy immediately after the injection of lofentanil.
The clinical studies showed that the sensitivity to lofentanil can vary greatly from person to person, which makes it difficult to determine suitable doses. Due to the extremely long duration of action and the difficult antagonizability (very high and repeated doses of naloxone are necessary), the substance is completely unsuitable for clinical use.
Miscellaneous and analogues
Lofentanil is the international non-proprietary name for levo cis -3-Methylcarfentanil ((-) - cis -3-Methylcarfentanil). However, in the literature, racemic (±) - cis -3-methylcarfentanil was apparently sometimes referred to as lofentanil. Since in some cases it is not clear whether the substance is racemic or optically active, dose information and information on potency should therefore be discussed with a certain degree of caution. (+) - cis -3-methylcarfentanil (R 34994, the enantiomer of lofentanil) has, in contrast to lofentanil, only a very low potency (ED 50 = 2.2 mg / kg and a short duration of action (at 2 × ED 50 : 30 min); for comparison, fentanyl = 0.011 mg / kg, lofentanil = 0.00059 mg / kg, carfentanil = 0.00032 mg / kg) and even shows partially antagonistic properties (partial agonist). For example, (+) - cis -3-methylcarfentanil antagonizes fentanyl-induced respiratory depression in rats with an ED 50 of 0.45 mg / kg (naloxone: 0.03 mg / kg).
(±) - trans -3-Methylcarfentanil (R 32812) has details of Janssen According to only 1 / 15 of the potency of (±) - cis -3-Methylcarfentanil and about 1.3 times fentanyl potency. According to Chinese studies, (±) - trans -3-methylcarfentanil (R 32812), on the other hand, is 0.85 times as potent as (±) - cis -3-methylcarfentanil.
Since a combination of cis -3-methyl group and beta-hydroxy group in fentanyl had an extremely strong effect on the potency ( ohmefentanyl ), some beta-hydroxy isomers and analogues of 3-methylcarfentanil were also investigated. According to Chinese information, beta-hydroxy- cis -3-methylcarfentanil (no further information on configuration, presumably a mixture of isomers) has about 2 times the potency of (±) - cis -3-methylcarfentanil and 1.3 times the potency of carfentanil ( Mouse, hot plate, ip). The National Institute on Drug Abuse (NIDA), on the other hand, specifies 0.7 times the carfentanil potency (mouse, hot plate, iv). Onset and duration of action should be similar to morphine. The substitution potency in opioid-dependent monkeys is the 6000 times of morphine and thus approximately 1 / 4 of the Carfentanils. The analogous ethyl ester ((±) - cis -ethyl {3-methyl-4- [N- (1-oxopropyl) -N-phenylamino] -1- (2-hydroxy-2-phenylethyl) -piperidine-4-carboxylate} ) has 30,000 times the morphine potency (substitution) in monkeys, but only reaches a maximum of 2900 times the morphine potency in mice, depending on the test method.
Replacement of the propionyl group by a cyclopropanecarbonyl residue in R 32792 increases the analgesic potency by a factor of 1.2 (mouse, hot plate, ip). Information on the duration of action and toxicity is not available. In the case of carfentanil, an analogous structural variation reduces the analgesic potency somewhat, while the toxicity is increased by a factor of 21 and the duration of action increases extremely and corresponds roughly to that of lofentanil (rat, tail withdrawal, iv).
Lofentanil was probably the substance with which Mossad agents attempted an assassination attempt on Hamas leader Chalid Maschal in 1997 , with an overdose being sprayed into his ear from behind. But other fentanyl derivatives are also being discussed, such as levofentanyl.
literature
- J. de Castro, A. Van de Water, L. Wouters, R. Xhonneux, R. Reneman, B. Kay: Comparative study of cardiovascular, neurological and metabolic side effects of 8 narcotics in dogs. Pethidine, piritramide, morphine, phenoperidine, fentanyl, R 39 209, sufentanil, R 34 995. I. Comparative study on the acute toxicity and hemodynamic effects of the narcotics in high and massive doses in curarized and mechanically ventilated dogs . In: Acta Anesthesiologica Belgica . tape 30 , no. 1 , March 1979, ISSN 0001-5164 , pp. 5-54 , PMID 382728 .
- J. de Castro, A. Van de Water, L. Wouters, R. Xhonneux, R. Reneman, B. Kay: Comparative study of cardiovascular, neurological and metabolic side effects of 8 narcotics in dogs. Pethidine, piritramide, morphine, phenoperidine, fentanyl, R 39 209, sufentanil, R 34 995. II. Comparative study on the epileptoid activity of the narcotics used in high and massive doses in curarized and mechanically ventilated dogs . In: Acta Anesthesiologica Belgica . tape 30 , no. 1 , March 1979, ISSN 0001-5164 , pp. 55-69 , PMID 474064 .
- J. de Castro, A. Van de Water, L. Wouters, R. Xhonneux, R. Reneman, B. Kay: Comparative study of cardiovascular, neurological and metabolic side effects of 8 narcotics in dogs. Pethidine, piritramide, morphine, phenoperidine, fentanyl, R 39 209, sufentanil, R 34 995. III. Comparative study of the acute metabolic toxicity of the narcotics used in high and massive doses in curarized and mechanically ventilated dogs . In: Acta Anesthesiologica Belgica . tape 30 , no. 1 , March 1979, ISSN 0001-5164 , pp. 71-90 , PMID 38620 .
- JE Leysen, PM Laduron: Receptor binding properties in vitro and in vivo of some long-acting opiates . In: Archives internationales de Pharmacodynamie et de Thérapie . tape 232 , no. 2 , April 1978, ISSN 0003-9780 , p. 243-246 , PMID 209755 .
- W. Gommeren, JE Leysen: Binding properties of 3H-lofentanil at the opiate receptor . In: Archives internationales de Pharmacodynamie et de Thérapie . tape 258 , no. 1 , July 1982, ISSN 0003-9780 , pp. 171-173 , PMID 6291471 .
- WE Meuldermans, RM Hurkmans, JJ Heykants: Plasma protein binding and distribution of fentanyl, sufentanil, alfentanil and lofentanil in blood . In: Archives internationales de Pharmacodynamie et de Thérapie . tape 257 , no. 1 , May 1982, ISSN 0003-9780 , pp. 4-19 , PMID 6214227 .
- LE Mather: Clinical Pharmacokinetics of Fentanyl and its Newer Derivatives . In: Clinical Pharmacokinetics . tape 8 , no. 5 , December 2012, p. 422-446 , doi : 10.2165 / 00003088-198308050-00004 .
- TF Meert, HR Lu, H. van Craenndonck, PA Janssen: Comparison between epidural fentanyl, sufentanil, carfentanil, lofentanil and alfentanil in the rat: analgesia and other in vivo effects . In: European Journal of Anaesthesiology . tape 5 , no. 5 , September 1988, ISSN 0265-0215 , pp. 313-321 , PMID 2905988 .
Individual evidence
- ^ D. Hank Ellison: Handbook of Chemical and Biological Warfare Agents, Second Edition . CRC Press, 2007, ISBN 978-1-4200-0329-1 , pp. 397 ( limited preview in Google Book search).
- ↑ a b Entry on Lofentanil. In: Römpp Online . Georg Thieme Verlag, accessed on August 24, 2016.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c d W.FM Van Bever et al .: N-4-Substituted 1- (2-Arylethyl) -4-piperidinyl-N-phenylpropanamides, a Novel Series of Extremely Potent Analgesics with Unusually High Safety Margin. Arzneimittel Forsch. 1976, 26, 1548. PMID 12771 .
- ↑ PGH van Daele et al .: Synthetic Analgesics: N- (1- [2-Arylethyl] -4-substituted 4-piperidinyl) N-Arylalkanamides. Arzneimittel Forsch. 1976, 26, 1521. PMID 12769 .
- ^ A b G. Van den Abeele, F. Camu: Clinical evaluation of the analgesic potency of lofentanil in postoperative pain . In: Acta Anesthesiologica Belgica . tape 34 , no. 1 , 1983, ISSN 0001-5164 , pp. 41-47 , PMID 6133403 .
- ^ A b H. Waldvogel: Analgesics, Antinociceptives, Adjuvants , Handbook for Pharmaceutical Practice, Springer (Berlin).
- ^ A b Alan F. Casy, Robert T. Parfitt: Opioid analgesics: chemistry and receptors . Plenum Press, New York 1986, ISBN 0-306-42130-5 .
- ↑ Patricia Maguire, Nancy Tsai, John Kamal, Chiara Cometta-Morini, Christopher Upton, Gilda Loew: Pharmacological profiles of fentanyl analogs at μ, δ and κ opiate receptors . In: European Journal of Pharmacology . tape 213 , no. 2 , March 1992, p. 219-225 , doi : 10.1016 / 0014-2999 (92) 90685-W .
- ↑ P. Bilsback, G. Rolly, O. Tampubolon: Efficacy of the extradural administration of lofentanil, buprenorphine or saline in the management of postoperative pain. A double-blind study . In: British Journal of Anesthesia . tape 57 , no. October 10 , 1985, ISSN 1471-6771 , pp. 943-948 , doi : 10.1093 / bja / 57.10.943 .
- ↑ a b c Wen, Sujei et al .: Synthesis an Analgesic Activity of Analogs of 3-Methyl-4-methoxycarbonylfentanyl. J. China Pharm. University 1992, 23, 196.
- ↑ NIDA Res. Mon. 1995, 152, 193.
- ↑ NIDA Res. Mon. 1995, 152, 197.
- ^ GA Brine et al .: Ohmefentanyl and Its Stereoisomers: Chemistry and Pharmacology. Curr. Med. Chem. 1997, 4, 247.
- ↑ Paul McGeough, Kill Khalid, Allen and Unwin 2009