Clear packaging
Under a blister pack - commercially internally as blister ( English , bubble bubble ') called - meant a product packaging that allows the customer or buyer to see the packed goods.
construction
The product is presented in front of a cardboard back wall , which is usually printed with information , and fixed with a plastic film molded part . The back wall of some transparent packaging is also made of plastic film, and for pharmaceuticals it is often made of aluminum foil .
A distinction is made between weldable packaging, clip-on packaging and staple packaging:
- In the case of welding packaging, the front of the film and the rear wall of the film are connected to one another by means of heat, which at the same time represents a product seal.
- With clip-on packaging, the edges of the front part of the film are bent around the back wall of the cardboard by heating the plastic.
- In the case of staple packaging, the front part of the film and the cardboard back are connected to one another using staples.
The shape of the blister contour is done using thermoforming technology .
In addition to the controversial customer benefits, the transparent packaging has advantages for the retailer. It can usually be pushed onto standardized holders (Euroslot), which facilitates presentation and inventory . The large packaging of small items makes shoplifting difficult.
Disadvantages from the point of view of environmental protection are the high level of packaging waste , especially in the case of small objects, and the poorer ecological balance of plastic compared to cardboard . Blisters are therefore controversial.
There are further disadvantages for the customer in particular: accessories can be hidden in cardboard inserts on the inside, so that there is only partial transparency. The packaging usually becomes unusable after first opening, making it difficult to return goods. Furthermore, the opening of blister packs made entirely of plastic is sometimes extremely difficult, since the front and back are usually welded together and it is very difficult to open them cleanly and easily without tools. In addition, cutting edges can cause injuries when opening the blister. After opening, the packaging remnants often take up a larger volume than when they were packed. This has an adverse effect on disposal. Because of these disadvantages, especially in handling, such packaging is rather unpopular with customers.
According to a press release dated November 3, 2008, Amazon.com decided to offer some products in alternative packaging with immediate effect and to gradually switch more items to more customer-friendly packaging in order to counteract the “frustration” of end customers caused by blister packaging.
Tablet packaging

A widespread use of blisters is in the packaging of tablets and capsules . Such a packaging is also called a push-through packaging. The cover film of a push-through strip that is not childproof is mostly made of hard aluminum film and is 20 micrometers thick. Arranging the tablets in individual recesses in the plastic or aluminum foil ( cavities ), which are sealed by the aluminum foil, has several advantages over glass or plastic bottles. It is more hygienic, undesirable influences such as high humidity (depending on the material) or dirt are excluded and the simple identification of the remaining number is very important. Special blisters allow the intake plan to be shown on the packaging. For example, the packaging of many birth control pills is printed with days of the week. This not only makes it possible to monitor the intake, but also to reliably assign different tablets to the days of the menstrual cycle .
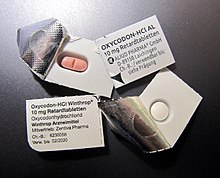
Narcotics such as morphine sulfate capsules or oxycodone are packed in blisters, the foil of which cannot be pushed through, but rather has to be pulled off with relatively great force . This serves to prevent accidental ingestion or as a child safety device .
The drug safety is only moderately supported by the lack of standardization of the incomplete labels on the blisters. As soon as a blister is removed from the outer packaging, its marking can no longer be fully identified or reliably assigned. This applies regardless of the system of labeling on the visual packaging.
To improve drug safety (better: therapy safety), the so-called pharmaceutical blister is used as part of pharmaceutical care . Almost the entire drug therapy can be individually packaged here according to the time of intake, provided that it is tablets or capsules.
So-called electronic blisters are used in clinical research. In this case, current-carrying conductor tracks are printed on the cover film in such a way that the conductor tracks lead over the express areas of a tablet in the blister. This “electronic blister” is connected to electronics that are arranged in a housing. As soon as a tablet is squeezed out of the blister, the conductor track is irreversibly torn. This change in state is recognized and processed by the electronics (e.g. display on a display). This invention goes back to a patent application from 1983 (DE 33 35 301 A). A further development of this system can be found in the patent application DE 10 2004 060 213 A1. In addition to being used in clinical research, electronic blisters are also used to improve compliance .
Web links
swell
- ↑ Amazon press release , November 3, 2008.
- ↑ Golem.de: Amazon unpacks: Simpler product packaging from Microsoft, Mattel and Fisher-Price , November 4, 2008.