humidity
The air humidity - or air humidity for short - describes the proportion of water vapor in the gas mixture in the air . Liquid water (for example raindrops, fog droplets) or ice (for example snow crystals) are therefore not included in the air humidity. The humidity is an important parameter for numerous technical and meteorological processes, for many life processes in living beings and for the health and comfort of people.
Depending on temperature and pressure, a given volume of air can only contain a certain maximum amount of water vapor. The relative humidity , which is the most common measure of humidity, is then 100%. In general, the relative humidity, expressed in percent (%), indicates the weight ratio of the current water vapor content to the water vapor content that is maximally possible for the current temperature and pressure. The air density is reduced by the absorption of water vapor , since with the total pressure remaining the same an added number of H 2 O molecules displace the same number of heavier N 2 and O 2 molecules.
humidity
Absolute humidity: Is in a given volume of air V water vapor mass contained m W . Usual unit : g / m 3 .
Maximum humidity: Is the maximum possible absolute humidity ( f max ) at a certain temperature . It is reached when the water vapor partial pressure in the air is as high as the saturation vapor pressure of the water at the corresponding temperature. In this condition the relative humidity is 100%. Usual unit: g / m 3 .
Relative humidity: Is the ratio of the actually contained to the maximum possible mass of water vapor in the air; Or, in other words, the relationship between the absolute humidity and the maximum humidity. As the quotient of two quantities with the same unit, this is a dimensionless quantity ; it is usually given in the auxiliary unit of measurement percent :
General
An air mixture free of water vapor is called dry air. Tables for the composition of the air generally relate to dry air, since the proportion of water vapor in humid air fluctuates very strongly with 0 to 4 percent by volume. The humidity is mainly influenced by the availability of water , the temperature and the degree of mixing of the atmosphere. Higher air temperatures allow a higher concentration of water vapor in the air. In the case of very low concentrations of water vapor in the air, the air humidity is also referred to as trace moisture or trace moisture .
Physical basics
Evaporation and condensation
On a free water surface that separates liquid water from the volume of air above, individual water molecules always pass from the water volume into the air volume. In liquid water, the water molecules are relatively strongly bound to one another by molecular forces, especially by the hydrogen bonds , which is what allows the coherent liquid bond to develop. As a result of their thermal movement, however, the water molecules each carry certain amounts of kinetic energy , which scatter around a temperature-dependent mean value ( Maxwell-Boltzmann distribution ). A small proportion of water molecules therefore always has enough thermal energy to overcome the binding forces of the surrounding molecules, leave the water surface and merge into the air volume, i.e. to evaporate . The evaporation rate , that is the amount of water evaporating per unit of time, depends on the proportion of those molecules whose kinetic energy exceeds the binding energy of the liquid compound and is determined, among other things, by the prevailing temperature.
Conversely, evaporated water molecules from the air also hit the water surface again and there, depending on their kinetic energy, can be captured by the molecular network with a certain probability, i.e. condense . The condensation rate only depends on the partial pressure of the water vapor in the air, but not on the proportion of the air pressure that the other components of the air supply.
Four variables influence the amount of this mass transfer:
- the size of the surface ( turbulence increases this value compared to still water),
- the temperature of the water,
- the temperature of the air and
- the degree of saturation of the air.
saturation
If you consider an evaporation process at constant temperature and initially dry air, the evaporation rate corresponding to the temperature occurs, while the condensation rate is initially zero due to the lack of water molecules in the air. The rate of evaporation is greater than the rate of condensation, and the number of water molecules in the air therefore increases. This also increases the condensation rate and the net evaporation (evaporation rate minus condensation rate) begins to decrease. The density of the water molecules in the air and thus the rate of condensation increase until the rate of condensation and rate of evaporation are the same, i.e. as many water molecules per unit of time pass from water into the air as from the air into the water. Then the equilibrium is reached in which the net evaporation is zero, although there is a constant exchange of particles between air and water.
The present at equilibrium concentration of water molecules in the air is the saturation concentration . If the temperature rises, a higher saturation concentration will set in, since the evaporation rate, which is now also increased, has to be compensated for by a higher condensation rate in order to achieve a new equilibrium, which requires a higher particle density in the air. The level of the saturation concentration therefore depends on the temperature.
The saturation concentration is determined almost solely by the properties of the water molecules and their interaction with the water surface; there is no significant interaction with the other atmospheric gases. If these gases were not available, practically the same saturation concentration would be established above the water. The colloquial language and, because of its simplicity, widespread expression in specialist circles, that air can absorb a maximum of a certain amount of water vapor at a given temperature is misleading. The air does not absorb moisture in the same way as a sponge, and the term saturation must not be understood here in the same way as the saturation of a solution . The air consists of independently acting gas particles that essentially only interact through impacts. So there is neither oxygen in the nitrogen nor water vapor in the other air components. (Imagine a closed container half-filled with water, in which there is a vacuum above the water surface. If kinetic energy in the form of heat is supplied to the liquid, particles with sufficient energy can detach from the surface (evaporate).) The saturation concentration is therefore dependent on the kinetic energy of the water particles.
For the same reason, the saturation concentration is not determined by the temperature of the air, but by the temperature of the evaporating surface. The reference to the temperature of the air is often justified in everyday practice, since evaporating surfaces with low thermal inertia usually approximate the air temperature (for example, laundry that dries in the air). However, if the evaporating surface is significantly warmer than the air, the water molecules evaporate into the cooler air (hot stove top) at an evaporation rate that corresponds to the surface temperature, even if their saturation concentration is exceeded. Part of the moisture then condenses in the air on the cooler aerosols that have assumed the air temperature and becomes visible as clouds of vapor or mist (for example, clouds of mist over an autumn lake). If the surface is much cooler than the air, the moisture content of partially saturated air can also lead to oversaturation and condensation on the surface (for example misted windows in the kitchen or bathroom or the increase in water in a pond ). More precisely, the water vapor condenses to water (to dew when the surface temperature is below the dew point , or to frost when it is below the frost point , see also below ).
Oversaturation
If the concentration of water molecules is increased above the saturation concentration ( supersaturation ), the condensation rate temporarily increases beyond the evaporation rate due to the greater density of water molecules in the air and the concentration of water molecules therefore falls back to the equilibrium value.
Here, too, it should be noted that it is not an inability of the air to hold the excess water vapor . Rather, the water vapor uses a present condensation surface under these conditions in order to lower its concentration to the saturation concentration through heterogeneous condensation . If such condensation surfaces or condensation nuclei are absent , the air can permanently absorb considerable amounts of water vapor until finally there is a spontaneous formation of water droplets ( homogeneous condensation ); see also the section on surface curvature of water. This is the case, for example, in large volumes of air that is as pure as possible, i.e. with a low aerosol concentration, and when the distance from any surrounding surfaces is great (see cloud chamber ). Spontaneous condensation of water vapor to water droplets takes place without condensation nuclei only when there is extreme supersaturation of several hundred percent relative humidity. In practice, however, there is almost always a sufficiently large amount of aerosols in the air so that there is hardly any oversaturation of several percentage points in the atmosphere.
Partial saturation
The rate of evaporation of the water cannot exceed certain maximum values. It therefore takes a long time for equilibrium to be restored after a disturbance. If, for example, part of the moisture content was condensed out by cooling down at night , the air is initially unsaturated after being heated and can only slowly return to saturation. This partial saturation is the normal case for our atmosphere because of the frequent temperature fluctuations. It is of great importance for numerous processes how far the air is from the state of saturation. Different moisture measures are used to describe this condition quantitatively.
Dependence of the saturation concentration on environmental influences
temperature
When the temperature rises, the proportion of water molecules increases, which have enough kinetic energy to leave the water surface . So there is a higher evaporation rate, which has to be compensated for by a higher condensation rate in order to restore the equilibrium, which however requires a higher concentration of water molecules in the air.
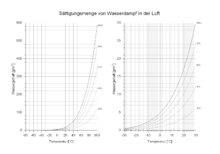
The saturation concentration of the water vapor therefore increases exponentially with increasing temperature , as shown in Figure 1 . The water vapor has a clearly defined saturation concentration for every temperature (and almost independent of the ambient pressure). At normal atmospheric pressure of 1013.25 hPa , one cubic meter of air at 10 ° C can absorb a maximum of 9.41 g of water. The same amount of air absorbs 30.38 g of water at 30 ° C and more than 100 g of water at 60 ° C. This saturation concentration is called the maximum humidity , which is tabulated in the article saturation . Here also are Mollier diagrams after Richard Mollier widespread (1923) to represent the humidity. Another way of showing the relationship between air humidity, temperature and altitude is the emagram .
pressure
As mentioned above, the saturation concentration of the water vapor at a given temperature is practically independent of the presence of the other atmospheric gases and thus almost independent of the ambient pressure. However, there is a slight dependence on the ambient pressure for three reasons:
- The water vapor and the other gases are not perfectly ideal gases. There are weak interactions ( van der Waals forces ) between their molecules, which increase with increasing pressure.
- The mutual distance between the molecules in the liquid water and thus their binding forces are slightly changed by the atmospheric pressure on them (“ Poynting effect ”). This in turn affects the rate of evaporation.
- Atmospheric gases dissolved in water also influence the binding forces and thus the rate of evaporation. The amount of dissolved gases depends on their partial pressure ( Raoult's law ) and thus ultimately on the total pressure.
This weak pressure dependency can be taken into account by a correction factor if necessary. It depends on temperature and pressure and is in the range of 0.5% under atmospheric conditions (details in the article saturation vapor pressure ).
State of aggregation of water
If one looks at an ice surface instead of a liquid water surface, the same considerations also apply to sublimation and resublimation of the water molecules. The ice cools the layer of air directly above it considerably, which means that it has a lower saturation concentration for water molecules. Sublimated water particles and the ambient air humidity therefore lead to the formation of condensation or mist in the vicinity of ice surfaces.
In the ice crystal association, however, the water molecules are subject to stronger binding forces than in liquid water, so that the saturation concentration over an ice surface is lower than over a surface of liquid ( supercooled ) water of the same temperature. This fact plays an important role in the formation of raindrops in clouds ( Bergeron-Findeisen process ).
Purity of water
| substance | relative humidity | source |
|---|---|---|
| Ammonium dihydrogen phosphate (NH 4 H 2 PO 4 ) at 23 ° C | 93% | |
| Potassium nitrate (KNO 3 ) at 38 ° C | 88.5% | |
| Potassium chloride (KCl) at 23 ° C | 85% | |
| Sodium chloride (NaCl) at 20 ° C | 75.5% | |
| Sodium dichromate (Na 2 Cr 2 O 7 • 2 H 2 O) at 23 ° C | 52% | |
| Magnesium chloride (MgCl 2 ) at 20 ° C | 33.1% | |
| Lithium chloride (LiCl) at 20 ° C | 11.3% |
If other substances are dissolved in the water, they make it difficult for the water molecules to leave the water surface, which reduces the evaporation rate and results in a lower saturation concentration (so-called dissolution effect ). In the air above saturated salt solutions, for example, the relative humidities listed in the table are established.
Although the air above the solutions is saturated with moisture, the relevant relative humidities are not 100%, since the relative humidity is always related to the saturation concentration over a flat and clean water surface (see below). If the air above the salt solution falls below the relevant saturation humidity, water evaporates from the solution in order to restore the saturation state. If the air exceeds the saturation humidity, part of the humidity condenses on the salt solution. This is diluted as a result; if it is to remain saturated with salt in order to maintain defined proportions, it must contain a sufficient sediment of undissolved salt.
The solution effect makes it clear again that the saturation concentration in the air is not determined by the air itself, but by the evaporating surface.
Surface curvature of water
If the surface of the water is convex (curved outwards), for example with a drop , the water molecules are less strongly bound to the surface and can leave the surface more easily. This curving effect means that the evaporation rate increases. When saturated air is in equilibrium with small mist droplets, its relative humidity is therefore a little over 100%. The same effect also means that strong supersaturation is possible without condensation nuclei, without homogeneous condensation occurring; Depending on the strength of the supersaturation, there is a certain minimum radius of the droplets below which they are not stable, since the evaporation rate increases with a smaller radius, but the radius decreases due to evaporation (see section critical radius under Kelving equation ).
If the water surface is curved inwards (as in the case of the meniscus in a partially water-filled capillary), the water molecules are more strongly bound to the surface and can leave the surface less easily - the evaporation rate decreases. When saturated air in a water-containing porous material is in equilibrium with the menisci, their relative humidity is less than 100%.
Moisture measurements
The water content of the air can be indicated by various so-called humidity measures. Designations that can be used synonymously are indicated by a slash, humidity measurements that belong together are in the same line.
- Vapor pressure (see also saturation vapor pressure ) and saturation deficit / hunger for steam ( Pa , hPa, kPa, bar )
- absolute humidity / water vapor density ( g / m³ , kg / m³)
- relative humidity ( % )
- specific humidity / water vapor content (g / kg , kg / kg)
- Mixing ratio / degree of moisture (g / kg, kg / kg)
- Dew point or frost point / ice point / frost point and dew point difference ( ° C , K )
- Wet temperature ( ° C )
Absolute humidity
The absolute air humidity , also water vapor density or vapor density for short ( symbol : ρ w , ρ d , d or a ; not binding), is the mass of the water vapor in a certain volume of air, i.e. its density or concentration . It is usually given in grams of water per cubic meter of air. It is limited at the top by the maximum humidity ρ w, max that prevails during saturation (see corresponding formulas and values there).
The absolute humidity is a direct measure of the amount of water vapor contained in a given volume of air. It shows immediately how much condensate can be deposited or how much water has to be evaporated in order to obtain the desired humidity.
The absolute humidity changes when the volume of the air parcel changes, even without water vapor being added to or withdrawn from the air. When the air package is compressed, the water molecules contained in it are concentrated in a smaller space, their number per cubic meter increases, the absolute humidity increases; the reverse applies to an expansion of the air parcel. The change in volume of the air parcel can be caused by changing its temperature or its pressure . When comparing the moisture content of two air parcels, their temperature and pressure differences should therefore be taken into account. A parcel of air rising in the atmosphere due to the thermals reduces its absolute humidity when ascending, even if it does not lose any water vapor in the process, as it increases its volume with the decrease in air pressure with altitude. The absolute humidity of the air parcel therefore changes solely through upward and downward movements. This is also referred to as displacement variance or unsteadiness . Since absolute humidity is also difficult to measure, it is rarely used.
The absolute humidity ρ w can be calculated using the following formulas, whereby the first term results from the conversion of the equation of state of ideal gases :
The individual symbols stand for the following quantities :
- e - vapor pressure
- R w - individual gas constant of water = 461.52 J / ( kg K )
- T - absolute temperature
- m water vapor - mass of water vapor within the air parcel
- V total - total volume of humid air
Table values see under saturation .
Relative humidity
The relative humidity (formula symbols: φ , f , U , RH , H or rF ; not binding) is the percentage ratio between the current vapor pressure of the water and the saturation vapor pressure of the same (at the air temperature) over a clean and level water surface. In the case of a non-percentage specification, i.e. in the value range 0 to 1, one also speaks of the saturation ratio .
The relative humidity shows immediately to what extent the air is saturated with water vapor:
- At a relative humidity of 50%, the air contains only half the amount of water vapor that could be contained at the corresponding temperature.
- At 100% relative humidity, the air is completely saturated with water vapor. It is also said that the " water vapor capacity " has been reached.
- If the saturation of 100% is exceeded, the excess moisture can precipitate as condensation water or mist .
Based on the relative humidity, it is easy to estimate how quickly evaporation processes will take place or how high the probability of condensation will be. Since the evaporation of moisture through the skin is largely determined by the relative humidity of the ambient air, the relative humidity is an important parameter for the feeling of comfort (see below).
A second reason for the importance of relative humidity is that it determines the balance water content of hygroscopic materials. Hygroscopic materials, especially porous materials such as wood, bricks, gypsum plaster, textiles, etc., absorb moisture when they come into contact with air and bind the water molecules by adsorption on their pore walls. The amount of bound molecules is determined by the absolute humidity on the one hand (a higher water vapor concentration leads to a higher adsorption rate because of the higher impact rate on the pore walls) and the temperature on the other hand (a higher temperature leads to a higher desorption rate ). The combination of these two opposing influencing variables means that the resulting balance water content is essentially determined by the relative humidity of the air. The moisture storage function of a material indicates what water content the material assumes at a given relative humidity; it is only slightly dependent on the temperature. To measure the moisture content of the air, materials are usually used, the physical properties of which depend on their water content (change in length due to swelling and shrinkage, change in capacitance of a hygroscopic dielectric , etc.). Since this water content is in turn determined by the relative humidity of the ambient air, such instruments therefore ultimately measure this relative humidity, which is therefore a particularly easy to measure and frequently used measure of humidity.
As the temperature rises, the amount of water vapor that would be required for saturation increases. As a result, the relative humidity of a given air parcel decreases when it is heated. The specification of the temperature is therefore essential for the comparability of the values. For example, in a desert that appears dry with an air temperature of 34.4 ° C and a relative humidity of 20%, a total of 7.6 g of water vapor is contained in one cubic meter of air, which is a relative humidity at an air temperature of 6.8 ° C of 100% and would therefore lead to condensation. Phenomena such as haze or fog are therefore a signal for high relative humidity and, at the same time, for low temperatures. The perception of the air as dry or humid is more due to the temperature than to the amount of water actually contained in it.
The relative humidity can be calculated using the following formulas:
The individual symbols stand for the following quantities:
- e - vapor pressure (attention, according to the definition of meteorology: see definition of vapor pressure )
- E - saturation vapor pressure
- ρ w - absolute humidity
- ρ w, max - maximum absolute humidity
- s - specific humidity
- S - saturation humidity
- μ - mixing ratio
- μ s - Mixing ratio at saturation
Deliquescence or deliquescence moisture describes the specific ability of a substance (mostly salts ) to influence the relative humidity of the surrounding air.
Specific humidity
The specific humidity (symbols: s , q or x ) indicates the mass of water that is in a certain mass of moist air. The range of numerical values is based on where is for dry air and is for air-free steam or liquid water .
In contrast to the previous moisture measurements, this variable remains unchanged when the volume of the air parcel under consideration changes, as long as no moisture is added or removed. Takes z. If, for example, the volume of the air parcel increases, both the (unchanged) mass of the moist air and the (unchanged) mass of the water vapor are distributed over a larger volume, but the ratio of the two masses in the air parcel remains the same. The specific air humidity, for example, maintains a constant value along a condensation-free ventilation pipe, even if the moist air runs through pipe sections of different temperatures or experiences pressure changes on its way, for example due to a throttle valve. A parcel of air rising in the atmosphere also retains the numerical value of its specific humidity as long as no humidity is added (e.g. through evaporation of raindrops) or removed (through condensation of the water vapor). However, this advantage is countered by the difficult measurement of the specific humidity, which is usually reserved for a laboratory.
The maximum specific humidity in the saturation state, the so-called saturation humidity , has the symbol S (also q s ).
The specific humidity s can be calculated with the following formulas, whereby the respective quantity is defined by the first term and all subsequent terms represent equivalents or approximations to this (fL - humidity air; tL - dry air; W - water vapor or water). Only the last-mentioned terms are of practical importance, all others are used for derivation and comprehensibility.
in order to:
where:
The saturation humidity is calculated according to:
The individual symbols stand for the following quantities:
- m x - masses
- ρ x - densities
- ρ fL - density of humid air
- V G - total volume of humid air
- R W - individual gas constant of water
- R tL - individual gas constant of dry air
- T - temperature
- M W - molar mass of pure water = 18.01528 g / mol
- M tL - molar mass of dry air = 28.9644 g / mol (value of the standard atmosphere )
- e - vapor pressure
- p - air pressure
- E - saturation vapor pressure
Mixing ratio
The mixing ratio (symbols: μ , x , m ), also known as the degree of humidity or water vapor content , indicates the mass of water that is in a certain mass of dry air . In terms of their properties, the mixing ratio and specific humidity are identical. As a rule, the numerical value does not differ very much either, which is why both quantities can be roughly equated.
The mixing ratio can be calculated with the following formulas, whereby it is defined by the first term and all subsequent terms represent equivalents or approximations thereto (fL - moist air; tL - dry air; W - water vapor or water):
The individual symbols stand for the following quantities:
- m x - masses
- ρ x - densities
- M W - molar mass of pure water = 18.01528 g / mol
- M tL - molar mass of dry air = 28.9644 g / mol (value of the standard atmosphere )
- e - vapor pressure
- p - air pressure
dew point
The dew point or dew point temperature is the temperature at which an equilibrium of condensing and evaporating water is established on an object (with existing moisture ), in other words the temperature below which condensation starts to form. It is measured with a dew point mirror hygrometer . The dew point of a sample only depends on the pressure, whereas the relative humidity is a variable that depends on pressure and temperature. The dew point is at a given atmospheric pressure for the respective temperature at the maximum value of humidity, can receive the air (= 100% relative humidity ). Cooling the air below the dew point temperature leads to condensation, warming to a new water vapor absorption capacity.
Wet temperature
The wet temperature is the temperature that an air parcel would have if it were adiabatically cooled to saturation at constant pressure by evaporation of water in the parcel and the required latent heat was extracted from the parcel. It is measured with the help of a psychrometer (for example Assmann's aspiration psychrometer ). If the temperature and humidity are known, the wet temperature can be read from a so-called psychrometer table. The formula for the wet bulb temperature is:
in which:
- T f - wet bulb temperature
- L - heat of phase change during condensation / evaporation (≈ 2450 kJ / kg)
- m - mixing ratio
- m s - saturation mixing ratio at wet temperature (!)
- T - abs. temperature
- c p - specific heat of air = 1005 J / (kg K)
Numerous empirical formulas have been developed in practical application, but most of them only work well in a certain temperature and pressure range.
In applied meteorology it is often used to differentiate between the type of precipitation (snow / rain) at unmanned weather stations. As a guideline, precipitation falls as rain at a wet temperature greater than or equal to 1.2 ° C, and as snow at Tf less than or equal to 1.2 ° C. However, only rough estimates can be made with this. The latest studies for the Vienna Hohe Warte station (WMO: 11035) have shown that precipitation occurs in solid or liquid form in 2/3 of the cases at Tf below 1.1 or above 1.4 ° C. In essence, the guideline value of 1.2 ° C wet temperature could be confirmed.
Measurement

Devices for measuring humidity are called hygrometers . Types are, for example, absorption hygrometers ( hair hygrometers ), psychrometers and dew point mirror hygrometers .
Moisture sensors deliver an electrical signal, absorption sensors are based on an electrical property of certain materials and material structures that changes with different water absorption . Examples of electrical sensors include impedance sensors, here it is the electrical conductivity that changes. With capacitive sensors, the moisture acts on the dielectric and thus changes the capacitance of the sensor; with quartz-based moisture sensors, the moisture changes the resonance frequency of the quartz.
Various measuring devices are used to measure air humidity in official weather stations around the world. One method is an aspiration psychrometer installed in the climate hut , which consists of a dry and a wet thermometer . The current relative humidity in percent and the dew point can then be determined from the values of both thermometers using a table . There are also separate measuring sensors for the dew point , which consist of a sensor over a lithium chloride solution .
Moisture indicators consist, for example , of silica gel (blue gel) mixed with cobalt chloride and change color at certain moisture values. They are used to enclose moisture-sensitive goods in order to be able to control their transport conditions with regard to the relative humidity, especially in tropical regions and when there are strong temperature differences. Blue gel (or the cobalt-free orange gel ) is also housed in hermetically sealed assemblies behind viewing windows in order to be able to control the humidity inside.
variability
Daily course
The air humidity shows a typical course of the day, which can be very different depending on the environmental conditions and does not always have to follow a certain pattern, but usually does. The following curve can be seen for Berlin in summer: at 7 a.m. local time, the average absolute humidity is around 10.6 g / m³, at 2 p.m. it is 10.0 g / m³ and finally at 9 p.m. it is 10 again. 6 g / m³. In winter the values are 4.5 g / m³ in the morning, 4.6 g / m³ at noon and again 4.5 g / m³ in the evening. The humidity increases in winter after sunrise and decreases after sunset with the daily change of the air temperature and in the way one can expect due to the increased evaporation. In summer there is also the influence of convection , as rising air parcels cause the penetration of drier air masses from above and therefore lead to a midday to afternoon minimum. In the evening hours, the absolute humidity increases again as the convection decreases. In summer there are therefore two vapor pressure maxima, one at around 8 a.m. and one at around 11 p.m.
The course of the relative humidity often reaches 100% near the ground at night (especially when there is no cloud cover), since the temperature of the air layers close to the ground falls below the dew point due to contact with the ground, which is cooling down through radiation into space. On windless days, the dew point on isolated horizontal surfaces (car roof, flat roof) falls below a short time (from 20 minutes) after sunset. It takes a little longer for vertical surfaces (car windows, traffic signs). The result is dew or frost .
Annual cycle
In the course of the year, based on either daily or monthly averages as long-term average values, maxima of the relative humidity appear in late autumn and early winter, i.e. in the period of greatest fog formation. In contrast, there are minimum values in spring and early summer. The vapor pressure is lowest in winter and highest in summer. The determining influences are evaporation and advection of water vapor, which have a very strong regional or local reference.
Depending on the height
The water vapor pressure decreases very quickly with increasing altitude and thus decreasing air temperature and then only slowly after three kilometers. At a height of ten kilometers it is then only about one percent of the land value. The relative humidity does not show such a clear trend , but is mostly very low in the tropopause , in Central Europe from about 11 kilometers above sea level. Here it is normally around 20% and continues to decrease with increasing altitude, which is also the reason that cloud formation is almost exclusively limited to the troposphere .
Significance and areas of application
Humidity is important in a large number of applications, with meteorology and climatology forming their theoretical but not their application-oriented center. The role of water vapor , its properties and in particular its technical applications outside of atmospheric conditions are explained there. The general properties of water and its natural distribution can be read separately.
everyday life
In everyday life, numerous phenomena can be traced back to air humidity, some of which are presented here as examples.
If you observe wet objects or open water surfaces over a longer period of time without additional water being supplied to them from the outside, their wetness decreases or the water surface dries out. Laundry becomes dry over time, puddles disappear, food becomes hard and inedible. Evaporation occurs . However, this is only possible as long as the air is unsaturated, i.e. the relative humidity is below 100%.
If you enter a heated room from a cooler environment, you will often notice that glasses are fogging up. The same applies to window panes. If the windows are colder than the interior, they fog up. This also restricts the field of vision in motor vehicles, for example. The same effect occurs in bathrooms and saunas , where mirrors and other colder objects often fog up. The reason for all these effects are the cold surfaces that cool the air in their immediate surroundings: the higher the relative humidity of the air, the faster it reaches the dew point when it cools and water condenses . The higher the temperature difference between the surfaces and the ambient air , the greater the tendency to condensation or fogging. For this reason, the cases described occur mainly in winter, in damp rooms, on external walls and outdoors at night with an uncovered sky (cooling of the earth's surface due to radiation into space). If the surface temperatures drop below 0 ° C, ice flowers or hoarfrost form . Countermeasures against condensation and tires:
- Blowing the panes with warm air
- Radiators in living rooms are located on external walls and under windows
- Heating of the objects (rear window of cars, aircraft components)
The effect also leads to freezing compartments and / or the evaporator in refrigerators and freezers, with simultaneous drying of unpackaged refrigerated goods. Their water first evaporates or sublimates in order to then condense on cold surfaces or to resublimate to ice . This effect is used technically in freeze drying .
The icing of the carburetors of gasoline engines (for example in motor vehicles or small aircraft) leads to engine failure. It is essentially based on the cooling of the air due to the evaporation cold of the gasoline, partly also due to the negative pressure, which additionally cools the air.
The dropping below the dew point can also be observed in airplanes or fast racing cars. The tip vortices at the ends of the wings or a spoiler lead to a local drop in air pressure and, according to Gay-Lussac's 2nd law, to local cooling of the air. The dew point is not reached locally and fog is created there. If the humidity is particularly high at temperatures below zero, the dreaded wing freezing occurs in aircraft - then the negative pressure above and behind the wings and tail units is enough to trigger tires.
In humans and homoiothermic animals, the exhaled air is much richer in moisture and warmer than the inhaled air. This can be recognized by the water vapor in the exhaled air condensing into visible clouds of fog in winter or at low temperatures and high humidity. The warm and moisture-rich exhaled air cools down below the dew point and water droplets are formed. The same applies to the exhaust gases from vehicles, aircraft and power plants, the formation of clouds or contrails of which are often confused with their pollutant emissions.
Meteorology, Climatology and Hydrology
If air saturated with water vapor is cooled below the dew point , then liquid water is separated from the air by condensation , if the necessary condensation nuclei ( aerosols ) are present. However, under natural conditions, these are almost always present in sufficient concentration, so that marked oversaturation of several percentage points only occurs in exceptional cases . The condensation and from temperatures below 0 ° C also the resublimation of the water vapor lead to the formation of clouds , hail , snow , fog , dew and frost , among other things . Water vapor is therefore not a permanent gas in the atmosphere and has a high mobility with a statistical residence time of around ten days.
Although water vapor is only present in relatively low concentrations in the atmosphere, due to its high mobility and the associated metabolism, it plays a large part in the global water cycle and therefore plays an important role in the water balance . The air humidity is also an important input variable for the formation of precipitation or its calculation and also for the determination of evaporation or evaporation , transpiration and interception evaporation . In the context of the climatic water balance, this in turn plays an important role for various climatic classifications .
Important meteorological parameters such as the level of condensation and the virtual temperature can also be derived from the humidity . The air humidity or water vapor also plays a major role in the radiation balance of the atmosphere - water vapor is the most important greenhouse gas . Water vapor, but especially clouds, greatly prevent the nightly cooling of the earth's surface, as they compensate for the radiation balance of the heat radiation from the earth's surface through absorption and re-emission .
The latent heat stored in the liquid state of aggregation of the water causes the difference between moist and dry adiabatic temperature gradients - one of the prerequisites for the development of foehn .
Drying
Air with a low relative humidity is a drying agent that is often used in everyday life , e.g. B. when drying textiles on the clothesline. When drying materials through evaporation, it is crucial that the air humidity is sufficiently low. At a relative humidity of 100%, the items to be dried cannot dry any further, an equilibrium is established. In drying processes , for example in dryers , including tumble dryers , attempts are therefore made to reduce the relative humidity of the environment. This can be done by increasing the temperature, exchanging air ( hair dryer , exhaust air dryer), by adsorbing the water (adsorption dryer) or by condensing the water (condenser dryer).
In other cases, however, the effect of the wind is generally relied on, which constantly blows in new air with a low relative humidity and thus removes the water, for example, hay , freshly felled wood, mortar , hanging laundry, tobacco leaves, coffee or cocoa beans.
biology
In biology and especially in ecology , the humidity is of great importance. It not only causes the occurrence of climatic zones or certain ecosystems , but also plays a major role in transpiration through the stomata of the leaves and in their intercellular space (water vapor partial pressure). The humidity is therefore an important parameter for the water balance of plants, animals and humans ( sweating , breathing, fungal attack). The humidity also plays a special role for those animals that mainly breathe through their skin. This includes many snails and other molluscs , which consequently also have a low tolerance to dehydration.
health
A relative humidity of 40 to 50% is recommended for living and office spaces. In cool areas, higher air humidity is more bearable than in particularly warm areas (below 20 ° C, over 70% can still be perceived as comfortable). Humidity levels above 95% and below 23% are generally uncomfortable. Under normal conditions, the air in heated rooms (in winter, especially when the outside temperature is low) can become too dry without active air humidification. On the other hand, the humidity in the bedroom should generally be slightly lower when the windows are closed, as the air humidity increases further through exhalation and the threshold for mold growth can be exceeded with an initial humidity of 60%. It is advisable to set up a hygrometer in the living rooms in order to measure the current humidity and, if necessary, to counteract this by means of regular burst ventilation or air dehumidifiers .
Causes and health risks if the humidity is too low
Particularly in closed, well-ventilated and well-heated rooms, the recommended values are often not reached, which can lead to reduced respiratory performance and impairment of the skin or mucous membrane . This is particularly the case in winter, as the cold outside air then has only a low absolute humidity and when it is heated to room temperature, the relative humidity drops very sharply. If the humidity drops too much, the unwanted exchange of air can be reduced by reducing leaks. However, even in the coldest areas of the room (outside walls behind furniture), the humidity should not exceed 80%, as mold growth cannot be ruled out at higher values . Depending on the use and thermal insulation of the rooms, humidity levels often result to avoid the growth of mold that are well below the medically recommended values.
In very cold areas or cold seasons or at night, the human organism often consumes more fluid, although the opposite should be assumed due to the lack of fluid loss through sweating. This is due to the humidification of the dry inhaled air and the associated loss of water. If the cold outside air is warmed up when it is inhaled, its water vapor capacity increases and the relative humidity is reduced. In contrast to this, the saturation deficit increases and the tendency of the liquid lung tissue water to change into the gaseous state of aggregation increases. In summer or when the ambient air is warm, the inhaled air is hardly heated any more and therefore retains its mostly high relative humidity. If the additional water losses through sweating are not too great here, the body's need for water is higher in cold ambient conditions.
If the air humidity is too low, it is not conducive to breathing , as the oxygen then reaches the bloodstream more poorly via the alveoli . The skin needs a high level of humidity in order not to dry out, as this is closely linked to the skin's moisture . Mucous membranes are particularly prone to drying out, as they have little protection against evaporation and are dependent on their high level of moisture to maintain their functions. A low level of moisture in the nasal mucosa can lead to an increased incidence of nosebleeds . In general, the immune defense of the skin is weakened (increased risk of colds) and its ability to exchange substances is reduced, which particularly affects the oral mucosa . The susceptibility to skin irritation or reddening or even skin inflammation is increased by a low level of humidity. If this inflammation only occurs in certain rooms or buildings, this is usually due to an additional pollution of the room air with pollutants (e.g. fine dust, solvents, formaldehyde, etc.).
When performing inhalation anesthesia, it is very important to moisten the inhaled gas mixture, as the medical gases used are stored in an anhydrous state and otherwise the evaporation effects in the patient's lungs would cause symptoms of cooling ( evaporative cold ) and a certain degree of dehydration.
Health risks if the humidity is too high
On the other hand, high relative humidity hinders the regulation of body temperature through sweating and is therefore quickly perceived as muggy . Despite higher temperatures, very hot deserts can therefore often be coped with much more easily by the organism (provided it does not suffer from dehydration) than rainforests with a high level of humidity and comparatively moderate temperatures. The effect of air humidity on the perceived temperature is described by the Humidex , whereby the fundamental relationship between increasing air humidity and increasing perceived temperature also applies to low values of air humidity and can thus be used, for example, to reduce the room temperature and thus the heating expenditure.
Agriculture and Forestry

In agriculture , if the humidity is too low, there is a risk of the fields and the crops being dehydrated, resulting in a bad harvest . By increasing the vapor pressure gradient between the leaf surface and the atmosphere, moisture is withdrawn from the plants (see section Biology), especially if their stomata are open during the day and they have little protection against evaporation, which is the case with many native plants (C-3 plants) , the case is. The plants thereby increase the drying out of the soil, on the other hand they protect it from direct sunlight and warming and through their roots promote water from deeper layers to the surface. Many bog and marsh plants have a control mechanism that lowers the evaporation rate when it begins to dry out.
In outdoor cultivation, the water balance is also significantly improved by nocturnal dew - plants are more likely to thaw than uncovered soil, as they cool down faster at night due to heat radiation than uncovered soil with its higher heat capacity .
But humidity also plays a role in forestry and the woodworking industry. Freshly felled wood has a high inherent moisture content; it is lower in wood felled in winter. This wood moisture drops during the period of deposition and adapts to the air humidity. If it is processed into fresh wood, it shrinks and warps. The change in wood moisture due to changing air humidity leads to changes in the dimensions of the wood across the grain, even with deposited wood, and is of great importance for all woodworking trades and industries. When storing fresh wood in sawmills , sprinkler systems are often used to dry the wood more slowly and thus avoid shrinkage cracks.
Also deposited wood ( boards , squared timber and beams ) is stored in such a way that air flows around it and is fixed in parallel by its own weight. This should guarantee that the wood does not warp or even rot . When laying plank and parquet floors, it must be ensured that the wood adapts to the ambient humidity due to its hygroscopicity. Below the fiber saturation range , this leads to swelling or shrinking of the wood. For this reason, wooden barrels also leak when not in use.
Warehousing and production

In the storage of food, the humidity is very important to control the consumption , especially with stored fruit . Also corrosion can be encouraged by high humidity, particularly be considered on the indirect effect of increased dew formation, and must therefore during storage and transport of moisture-sensitive goods. Examples that require certain humidity levels are chemicals , cigars (humidors), wine (corks), salami , wood , works of art , books and optical or electronic assemblies and components, for example integrated circuits . The air humidity must be monitored or controlled in order to maintain certain indoor climates in storage rooms , museums , archives , libraries , laboratories , data centers and industrial production systems ( microelectronic production).
When transporting goods in weather-insulated containers or welded plastic bags , condensation and condensation can form if the air inside falls below the dew point when the temperature drops, for example when transporting from tropical to colder areas. In film packaging of moisture-sensitive goods, bags with silica gel or zeolites are used to buffer the moisture. Moisture indicators are used to monitor the moisture levels in the packaging during transport. Moisture-sensitive devices such as B. in electronics and optics must first temper after storage at low temperatures before their packaging is opened. Otherwise, condensation will form on and in the devices, which can lead to failure, especially if the devices with condensation are operated immediately.
Exterior walls of buildings
In building physics , the dew point in the form of the dew point level plays an important role. This is understood to be the area within the masonry or the thermal insulation on the outer wall of a building from which condensation can occur. The reason is that warm air can absorb more moisture than cold air. If warm and moisture-enriched air moves through diffusion or convection within the outer wall or insulation layer from a warmer to a colder place (in winter mostly from inside to outside), liquid water forms as soon as the dew point is fallen below. This results in the risk of mold formation , which is hazardous to health, or the insulation layers fail due to water absorption (better heat conduction ) or from frost bursts (for "diffusion" and "diffusion-open building materials" see breathing wall ).
Countermeasures therefore consist of avoiding falling below the dew point by using suitable building materials or other measures. The thermal insulation should therefore be attached to the outside of the wall as far as possible and should in turn be open to diffusion to the outside so that it can release water into the dry outside air. If this is not possible (for example with interior insulation), the thermal insulation layer must be provided on the inside with a vapor barrier (closed film, no water diffusion possible) or vapor barrier (water diffusion is possible to a limited extent) in order to prevent moist room air from penetrating the thermal insulation layer. This is particularly important if the masonry has a low diffusivity, for example due to an external coating.
In addition, an insulation layer can also be wetted from the outside . Dew or other precipitation (for example clinker facing clinker stuck in the joints ) can be sucked in by capillary action if there are stress cracks or shrinkage cracks . If the interface between the thermal insulation and the outside air is impervious to liquids or steam and there is no ventilation , moisture that has penetrated can no longer dry out and the insulation material wets over a large area and irreversibly (see also moisture # moisture in building components )
During the winter period - in this context often referred to as the thaw period - the temperature and the water vapor pressure inside are higher than outside. The outer wall therefore has an outward gradient for both values. However, this is not the same even with a homogeneous outer wall, since their time-dependent storage effect for heat and water vapor is different and the temperatures and vapor pressures also change differently over time. In the case of inhomogeneous walls, there is also the fact that the gradient in the individual materials is different. For example, a vapor barrier film has a large vapor pressure gradient, but hardly a temperature gradient. In the case of insulation materials, it is often the other way round, here the water vapor pressure gradient is small, but the temperature gradient is high. Condensation always occurs when the local relative humidity temporarily or (for example in winter) permanently exceeds 100%.
The formation of condensation can also be prevented by building materials with high water vapor permeability and / or a high water absorption capacity (buffering) with low thermal conductivity at the same time. Examples are straw / clay or wood. Vapor barriers can often be dispensed with here.
Proper ventilation of living spaces (especially when renovating with exterior paint, improperly installed vapor barriers and sealed windows) has a major impact on the prevention of mold growth.
See also: low-energy house , building biology .
According to the architect and specialist book author Konrad Fischer , with radiant heating the "building envelope surfaces" would always be warmer than the air by absorbing heat radiation, the air there would never be cooled below the dew point , the interior plaster could not become wet and mold would not grow; with convection heating , however, the air would always be warmer than a wall that could be causing fallen below a wall of the dew point., Fischer took over doing essentially the theses of the architect Claus Meier it "the room air temperature can substantially lowered at a radiation-intensive heating over the konvektionsoptimierten heaters - The energy saving is therefore enormous « . Because the humidity of the indoor air (sources: breathing, evaporation, drying, plants, etc.) is to be removed from living spaces by air exchange, more energy would be lost with the air exchange with convection heating (in which the room air is heated) than with body or traveling warming Radiant heater. Thus (according to Fischer) heating systems based on thermal radiation, even with leaky windows, would be more efficient than those with convection heating and thermal insulation. Failure to change the air in the pleasantly warm air would often lead to condensation of moisture in interiors and, as a result, in connection with organic nutrients (from binders, paint, wallpaper adhesives, paper wallpaper) to serious black mold pollution.
Aerospace
In aviation, there is a risk of the wings and tail unit icing up due to the resublimation of the water vapor contained in the air. This effect can severely limit the ability to fly within a very short time and is responsible for numerous accidents. This process is counteracted by de-icing systems, which heat the critical areas (e.g. leading edge of the wing) in order to prevent ice accumulation.
A cheaper method is to cover the leading edge of the wing with a skin made of rubber and pressurized compressed air between the rubber skin and the wing. The skin bulges and the deformation breaks off the rigid ice. However, this method carries a certain risk. If the resulting ice shell is still thin at the time the compressed air defrosting is triggered, the rubber skin only arches it, but does not blow it up. As a result, more ice builds up and the re-triggering of de-icing remains inconclusive. To counteract this risk, pilots often wait to operate the de-icing until they are of the opinion that it can actually achieve the desired effect.
In space travel , rocket launches have similar problems caused by low outside temperatures. Start windows are therefore also selected according to meteorological aspects and starts are canceled if necessary. Failure to observe this principle can lead to a crash.
Respiratory protection
The air humidity is an important parameter when filling compressed air cylinders from z. B. Compressed air breathing apparatus. For this purpose, the air humidity according to DIN EN 12021 "Compressed air for breathing apparatus" is specified as the maximum water content of the air stored in compressed air cylinders and the air measured at the outlet of the compressor, i.e. the absolute air humidity a, d or f.
According to DIN EN 12021 compressed air for breathing apparatus, the maximum water content in compressed air cylinders may be:
- at 200 bar nominal pressure: 50 mg / m 3
- at 300 bar nominal pressure: 35 mg / m 3
The absolute humidity of the air supplied by the compressor for filling 200 bar or 300 bar compressed air cylinders should not exceed 25 mg / m 3 . The air humidity is measured in the respiratory protection with test tube measuring devices . The unit of measurement relates to air relaxed to atmospheric pressure.
Heat exchange
On heat exchangers and cold pipes that are colder than the ambient air, condensation of air humidity and, if the temperature drops below freezing point, also icing can occur.
Condensation occurs inside a refrigerator, which is therefore usually operated just above freezing point. Formerly (around 1960/1975) the - only - cooling surface as a horizontal plane made of anodized aluminum formed the bottom of the freezer compartment and was thus shielded over the refrigerator compartment. The cooling surface iced up with the humidity coming from the room air and from water-containing foods and therefore had to be defrosted about weekly . The ice then melted and either dripped into a device made of roof-shaped and channel-shaped webs, which was constantly inserted in the refrigerator, into a collecting tray that had to be pulled out by hand and emptied. Later devices, no longer insulated with glass wool, but better insulated with foam, had a continuous tub made of plastic with a drainage nozzle at the rear in the refrigerator, the stubble of which is opened for defrosting in order to allow the defrosted water to be drawn into a container below. Since around 1980, the rear wall, seamlessly made from blown plastic, has formed the cooling surface of the cold room. Here condensed water - possibly temporarily frozen during a cooling phase - runs down into a molded groove and further through an always open outlet, into a plastic cup on the outside of the warm cooling unit and evaporates there. Such refrigerators are self-defrosting. The freezer compartment, which is largely airtight and thus almost water-vapor-tight, is closed by plastic beads filled with magnetic strips and is only rarely opened and therefore only a little ice builds up on its own cooling surface, which has to be defrosted manually.
When the dew point of the air in the cellars of houses rises in summer, the air moisture condenses on the pipe of a drinking water pipe through which it flows.
A number of gases (propane, butane, CO 2 , nitrous oxide) are liquefied under pressure and kept in stock in pressure bottles, cartridges or small metal cartridges. Quantities withdrawn from the gas phase at a sufficiently high rate are replenished from the liquid phase by evaporation or boiling, which cools it down, which leads to liquid condensation of humidity on the outside of the upright bottle and, if the ambient temperature is sufficiently low, to frost formation, which is visible in the mirror height of the liquid phase of the contents.
If compressed air that has not been specially dehumidified is released from a boiler quickly, the air in the jet cools down during relaxation so that the ambient air that is entrained can be cooled below its dew point, so that a little fog forms temporarily and locally. A similar effect occurs when an upright container of a drink is opened quickly, which contains carbon dioxide under a certain pressure. If the drink does not foam out, a small cloud of mist will briefly be visible over the opening of the bottle or can.
Drinks poured cold in drinking glasses cause humidity to condense outside. In order to protect tables, beer mats are placed underneath. Stem glasses usually keep the stem dry as long as the coating of fine drops has not clumped together to form larger ones that run off. About stems from Pils tulips are often Pilsdeckchen slipped that abrinnenden foam and condensate are to absorb.
Air conditioning systems mounted on external walls allow water to condense in the cooled air flow. Small amounts of liquid water are sometimes conducted through small pipes onto the sidewalk in front of a shop.
Dehumidifying and drying air and fabrics
Dehumidifiers down to the size of a travel bag work by cooling blown air to below the dew point, draining the water condensed on the cooling surfaces into a collecting vessel and more than reheating the air. The compressor chiller is typically driven by an electric motor.
The use of hygroscopic substances (solid, rarely liquid) is only recommended for small air volumes. Electronic devices, but also leather goods susceptible to mold, are packed in small paper bags of dried silica gel in order to bind a certain amount of moisture that diffuses through cardboard packaging during sea transport in containers and can condense when it cools. Tissue paper or the like is often packed as an intermediate layer between water vapor-tight layers of glass or plastic film and the like in order to promote the exchange of moisture in order to avoid liquid condensation and the associated transport processes and capillary effects.
In the chemical laboratory, substances are often required anhydrous in order to weigh them without water content or to process them without water. The drying takes place roughly in air, more or less sharply by heating, possibly until glowing. Humidity causes water to be absorbed again when it cools down. That is why substances are stored in bowls in the desiccator next to or above desiccants. The substance to be dried releases - at room temperature - water vapor as humidity and z. B. silica gel, calcium chloride or concentrated sulfuric acid absorbs the water vapor due to its higher hygroscopicity . Air is usually sucked out of the desiccator with a water jet pump, which facilitates the escape of water vapor (and other vapors) from the sample and the diffusion of the water vapor towards the desiccant. By creating a vacuum of down to about 1/100 bar, the absolute humidity increases up to a hundredfold. If, for example, water at ambient temperature (e.g. 20 ° C) is present in the desiccator as a source of water vapor, the relative humidity does not change after equilibrium has been established. Because the water vapor pressure at 20 ° C ( ideally considered) always causes saturation with water vapor, i.e. 100% relative humidity, regardless of the air molecules that are also present in the same volume.
A water jet pump is expediently operated with cold water, since it represents a source of water vapor at the temperature of the pump in the direction of a vacuum. On the desiccator, it is only used intermittently and not for a long time to extract organic vapors (e.g. solvents).
With freeze-drying, frozen food, often food, is dried gently in a vacuum because it is not heated. Evaporating water vapor is sucked in under vacuum. Flavors that are less volatile than water or that adhere more strongly to the substance are retained.
literature
- H. Häckel: Meteorology. (= UTB. 1338). 4th edition. Ulmer Verlag, Stuttgart 1999, ISBN 3-8252-1338-2 .
- E. Zmarsly, W. Kuttler, H. Pethe: Meteorological-climatological basic knowledge. An introduction with exercises, tasks and solutions. Ulmer Verlag, Stuttgart 2002, ISBN 3-8252-2281-0 .
- P. Hupfer, W. Kuttler: Weather and climate. Teubner, Stuttgart / Leipzig 1998, ISBN 3-322-00255-1 .
- W. Weischet: Introduction to General Climatology. Borntraeger, Berlin 2002, ISBN 3-443-07123-6 .
Web links
- Information on the role of humidity for wood and the indoor climate
- Manfred Reiber: The importance of humidity for flying and ballooning. Article with an extensive general part on humidity, (PDF file; 769 kB) , private website
Individual evidence
- ^ A b Julius F. von Hann: Handbuch Der Klimatologie. 1st edition. Salzwasser Verlag, 2012, ISBN 978-3-86444-581-1 , pp. 44-50.
- ↑ Jochen Harsch: Schimmel - causes and connections . epubli, Berlin 2014, ISBN 978-3-7375-0741-7 .
- ↑ Jürgen Schatz, Robert Tammer (Ed.): First aid - chemistry and physics for physicians. 3. Edition. Springer Verlag, Berlin / Heidelberg 2015, ISBN 978-3-662-44110-7 .
- ^ Rainer Müller: Thermodynamics. From dewdrops to solar power plants. Walter de Gruyter, Berlin 2014, ISBN 978-3-11-030198-4 .
- ^ Alfred Dengler: Silviculture on an ecological basis. A teaching and manual. 3. Edition. Springer Verlag, Berlin / Heidelberg 1944.
- ^ SA Bell, SJ Boyes: An Assessment of Experimental Data that Underpin Formulas for Water Vapor Enhancement Factor . National Physical Laboratory, UK, 2001. ( online access ).
- ↑ a b c d DIN 52615: Determination of the water vapor permeability of construction and insulation materials. Berlin 1987.
- ^ A b c L. Greenspan: Humidity Fixed Points of Binary Saturated Aqueous Solutions. In: Journal of Research of the National Bureau of Standards - A. Physics and Chemistry. Vol. 81 A, No. 1, January-February 1977, pp. 89-96. ( PDF ; 320 kB).
- ^ Friedrich Waidacher: Handbook of general museology. 3. Edition. Böhlau Verlag, Vienna / Cologne / Weimar 1999, ISBN 3-205-99130-3 , pp. 396-399.
- ^ Réné Du Bois-Reymond: Physiology of humans and mammals. 4th edition. Springer Verlag, Berlin / Heidelberg 1920, pp. 80–82.
- ^ RE Huschke: Glossary of Meteorology. American Meteorological Society, Boston 1959.
- ↑ J. Rohrgger: Methods for determining the snowfall line. Thesis. Institute for Meteorology and Geophysics at the University of Vienna, 2008.
- ^ Herbert Maria Ulrich: Handbook of the chemical investigation of textile fibers. First volume, Springer Verlag, Vienna 1954.
- ↑ a b c Fachverband Gebäude-Klima e. V .: indoor air humidity . P. 4.
- ↑ A pleasant humidity promotes health . wallstreet-online.de guide. Retrieved January 31, 2011.
- ↑ Healthy humidity . German Green Cross - Environment and Health. Retrieved January 31, 2011.
- ↑ Climate in the office , Ergo Online, accessed on January 31, 2011.
- ^ W. Petro (ed.): Pneumological prevention and rehabilitation. 2nd Edition. Springer Verlag, Berlin / Heidelberg 2000, ISBN 3-642-64112-1 .
- ↑ Wolfgang Oczenski (Ed.): Breathing - Respiratory Aids . Respiratory physiology and ventilation technology. 8th, revised edition. Georg Thieme Verlag, Stuttgart 2008, ISBN 978-3-13-137698-5 .
- ^ Josias Braun-Blanquet: Plant sociology . Basics of vegetation science. Springer Verlag, Berlin / Heidelberg 1928.
- ↑ Bernd Wittchen, Elmar Josten, Thomas Reiche: Holzfachkunde. 4th edition. Teubner Verlag, Wiesbaden 2006, ISBN 3-519-35911-1 .
- ↑ Ecotrophology 2 . 1st edition, Verlag Neuer Merkur, Munich 2005, ISBN 3-937346-03-1 .
- ^ Johann Hamdorf, Heribert Keweloh: Management systems for food safety. DIN EN ISO 22000 in practice. 1st edition. Beuth Verlag, Berlin 2009, ISBN 978-3-410-16826-3 , pp. 16-17.
- ↑ Horst Bieberstein: Mold in living rooms - what to do. 3. Edition. Bieberstein Alpha and Omega Verlag, Stuttgart 1995, ISBN 3-927656-06-2 .
- ↑ Michael Köneke: Recognize mold in the house - avoid - fight. 3rd, revised edition. Fraunhofer IRB Verlag, Stuttgart 2008, ISBN 978-3-8167-7295-8 , pp. 17-18.
- ↑ Prof. Meier's controversial contributions to energy saving 5
- ↑ Konrad Fischer: The temperature control of the building envelope surfaces 21
- ↑ a b Meier, C .: Practical Guide to Monument Preservation No. 7, Old Buildings and Thermal Insulation - 13 questions and answers. Information publications of the Deutsche Burgenvereinigung eV, Marksburg, Braubach, 1999; cited in The temperature control of the building envelope surfaces 21 .
- ↑ Prof. Dr.-Ing. habil. Claus Meier: Are we insulating ourselves in the dead end? Thermal insulation and energy saving ordinance. Contradictory and absurd , lecture on the occasion of the Backsteintage 2001, January 30/31, 2001 in Hildesheim / Westerstede, (PDF file)
- ↑ Niels Klußmann, Arnim Malik: Lexicon of aviation . Springer Verlag, Berlin / Heidelberg 2004, ISBN 3-540-20556-X .
- ↑ Compressed air for breathing apparatus atemschutzlexikon.de, accessed on March 16, 2017.