Airtightness
The air density ρ (also density of air or density of air ) indicates the mass of air contained in a certain volume . At sea level , the air, at around 1.2041 kg / m³ at 20 ° C, is more compressed by the air mass above than at higher altitudes: The air is therefore relatively denser.
Altitude dependence
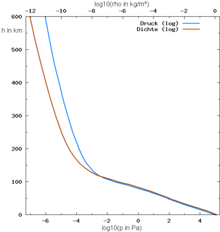
The air on the ground always has the highest density and the highest air pressure and - except for inversions - also the highest temperature; at higher altitudes the air becomes thinner and thinner.
If the temperature were the same at all altitudes, the air pressure and air density would also decrease together with increasing altitude according to the gas law (see barometric altitude formula ). However, the temperature at different altitudes varies greatly.
It is not exactly true that the pressure and density of the air should theoretically decrease by half for every 5000 meters:
- 90% of the atmosphere is below 20 km.
- 70% of the atmosphere is below 10 km.
- 55% of the atmosphere is below 5 km.
calculation
If air is considered the ideal gas , the air density ρ in kg / m³ is calculated as:
With
- the air pressure p in pascals ; Atmospheric air pressure p 0 = 101325 Pa = 1013.25 hPa = 1013.25 mbar = 1.01325 bar .
- the molar mass M (in SI units )
- the universal gas constant R = 8.3145 J / (mol · K) with the energy in J (= N · m )
- the temperature T in Kelvin ; { T in Kelvin} = {temperature in ° C} + 273.15.
By inserting the specific gas constant for dry air one obtains:
- .
Temperature dependence
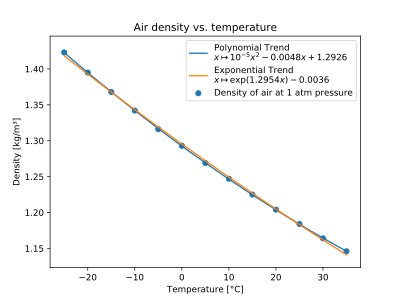
| Temperature in ° C
|
Temperature T in K |
Air density in kg / m³
|
annotation |
|---|---|---|---|
| +35 | 308.15 | 1.1455 | |
| +30 | 303.15 | 1.1644 | |
| +25 | 298.15 | 1.1839 | |
| +20 | 293.15 | 1.2041 | Laboratory conditions |
| +15 | 288.15 | 1.2250 | Aviation standard atmosphere |
| +10 | 283.15 | 1.2466 | |
| +5 | 278.15 | 1.2690 | |
| 0 | 273.15 | 1.2920 | physical standard conditions |
| −5 | 268.15 | 1.3163 | |
| −10 | 263.15 | 1.3413 | |
| −15 | 258.15 | 1.3673 | |
| −20 | 253.15 | 1.3943 | |
| −25 | 248.15 | 1.4224 |
Humidity dependence
An exact determination of the density of the air requires the air humidity to be taken into account , as this changes the gas constant of the air:
With
- the gas constant of dry air
- the gas constant of water vapor
- the relative humidity (e.g. 0.76 = 76%)
- the saturation vapor pressure of water in air, which inWikibooks: data table with more precise formulas - learning and teaching materialsis tabulated. There are also empirical formulas of varying accuracy such as the Magnus formula or Antoine equation .
- the ambient pressure in Pascal.
After adjusting the gas constant, the equation
be used.
Exact determination
In order to minimize measurement errors , we recommend an aspiration psychrometer to determine the air humidity and a mercury barometer to determine the ambient pressure . The barometer reading still has to be corrected for capillarity , the summit height of the mercury level, temperature-dependent density of the mercury and local acceleration due to gravity .
Reciprocal
The reciprocal value of the density, the specific volume , has the symbol α in meteorology and the symbol v air in thermodynamics :
- .
Web links
- Calculator for air humidity parameters Calculates the air density and other data of the air online if the temperature, humidity and air pressure are entered.
Individual evidence
- ↑ The universal gas constant. Retrieved December 21, 2019 .