capillarity

Capillarity or capillary effect (Latin: capillaris , relating to the hair) is the behavior of liquids when they come into contact with capillaries , i.e. sufficiently narrow tubes, gaps or cavities in solids. The effect is caused by the interaction between the surface tension of the liquid ( cohesion ) and the interfacial tension between the liquid and the solid surface ( adhesion ). Since the weight of the liquid is low in narrow cavities, which predominates capillary force against the gravity and helps about trees going to let water ascending up from the roots up to 100 meters (see water transport in plants ).
Capillarity causes liquid wax in the wick to rise to the flame and porous materials such as bricks , textiles and paper soak up water
In non-porous material there must be fine gaps in order to allow water to rise. So water rises in a narrow glass tube and in enough fine sand against the gravitational force.
Effects
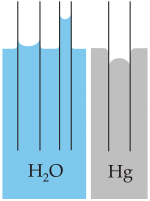
Capillary ascension (ascent) occurs with liquids that wet the material of the capillary , such as water on glass or on paper fibers. The water rises in a glass tube and forms a concave surface ( meniscus ). This behavior is due to the adhesion force (force that acts between two substances).
Capillary depression (descent) occurs when the liquid does not wet the material of the vessel surface. Examples are mercury on glass or water on a greasy surface. Such liquids have a lower level in a tube than in the surroundings and have a convex surface.
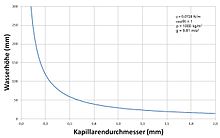
Up to a certain minimum diameter, the smaller the diameter of the capillaries, the greater the capillary pressure and the rise, see formula and table below.
Sponges and wipes have great absorbency as long as their structure can withstand the capillary suction and does not collapse.
In a tube, the liquid rises due to adhesive forces on the wall of the tube and thus only to its end, even if the capillarity allowed a greater height of rise.
In a tapered tube, surface tension drives a film of liquid towards the smaller diameter, which in the case of a pipette promotes emptying.
A vessel can empty completely if a rag hangs down on either side of the rim. The liquid rises in the textile through capillary force, passes over the edge and is distributed throughout the entire cloth. The liquid outside the vessel is pulled down by gravity and sucks the liquid in the vessel through the siphon effect in the capillaries as soon as it is sufficiently deep below the liquid level in the vessel.
A coarse-grained, capillary-breaking layer prevents rising damp in buildings and is installed as a frost protection layer under road surfaces.
Molecular Consideration
Basically, the effect of capillarity is based on the molecular forces that occur within a substance ( cohesive forces ) and at the interface between a liquid, a solid (vessel wall) and a gas (e.g. air) ( adhesive forces ). Often the capillary effect also has the meaning of surface tension .
Inside a body, the forces acting on a particular molecule from its environment cancel each other out. At the edges, however, there is a resultant force which, depending on the material in question, is directed either into or out of the liquid. If the effect of the vessel wall against the cohesive forces in the liquid is small, then the resulting force points into the interior of the liquid. Its surface is curved downwards at the point of contact with the wall and does not wet the vessel wall (e.g. mercury in the glass vessel). However, if the effect of the vessel wall against the cohesive forces in the liquid is great, then the resulting force points into the vessel wall and the liquid is bent upwards at the edge. The liquid wets the wall (e.g. water or petroleum in a glass vessel).
Practical applications
Fountain pen : An example of an application is the fountain pen or fountain pen or its nib . As a rule, it has a small, round hole halfway along its length, in which the ink collects in order to be transported from there by capillary action through a very fine slot to the tip.

Paper: paper absorbs the ink through the capillary effect; it is even possible to write upside down. Writing with a fountain pen is hardly possible on smooth surfaces such as glass, as the ink only wets the surface of the glass but is not absorbed.
Plants: In trees and other plants, the water is absorbed by the roots and then transported to the crown, where it evaporates from the stomata of the leaves (or needles) or is required for photosynthesis . The evaporation in the upper area of the plant causes a perspiration suction , cohesive forces of the water in the plant prevent the flow of liquid from breaking off, and the capillary effect with the osmotic effect ( root pressure ) favors the ascent. According to new findings, trees can reach a maximum height of 130 meters, because then the osmotic pressure together with the capillary forces is no longer sufficient to overcome gravity . See water transport in plants
Chemistry: In paper chromatography , the capillary effect is used in that a solution is dripped onto a special paper and rises up on it, with components of the solution being carried along. Due to the different spacing, the fabrics can be separated.
Medicine: In order to draw off small amounts of blood, you can make a small prick in the vessels on the fingers or the earlobe and hold a thin collection tube in which the blood rises due to the capillary effect and can thus be collected.
Textiles: A similar suction effect as with paper can also be observed with cleaning rags or fabrics. The same goes for sponges . For paper, cleaning rags and sponges, the following applies: the larger the inner surface (per volume), the greater the suction effect.

Soldering: The same effect occurs during soldering : the liquid solder flows through the capillary action into the gap in copper pipe fittings, for example . A wire mesh, the desoldering braid , is often used to desolder electronic components from printed circuit boards .
The quality of the soldering result is immediately recognizable from the shape of the soldering cone. If this is not concave and tapering flat on the board, it is most likely a cold solder joint . Because of the capillarity, “overhead” soldering is also possible.
Construction: In construction, capillarity plays an important role. Most of the measures for building waterproofing are directed against the capillary rising moisture in the floor slab and walls . In the case of above-ground components, on the other hand, capillary building materials have the welcome effect of distributing moisture accumulations over a large area. If the moisture reaches a component surface, it can evaporate. Through the so-called transpiration suction , water is then continuously fed until the equilibrium moisture content is reached. Capillary building materials can also absorb and distribute larger amounts of water that have dampened ceilings and walls as a result of a broken pipe , for example , before structural damage and mold formation occur.
In winter, the dew point of the indoor air is usually below the dew point of the indoor air at cold spots on the outer wall , so that condensate is formed, which evaporates during ventilation and is released into the outdoor air. If too much condensate forms or if too little ventilation is provided, the moisture will collect in the wall. Walls and ceilings, which consist entirely of capillary-active building materials, can absorb the moisture and direct it to the outside of the wall or into rooms with less moisture, where it evaporates.
Traditional bricks and sand-lime bricks are very absorbent ; hard-burned bricks ( clinker ), aerated concrete and concrete have a significantly lower capillary effect . In order to interrupt the capillary flow in buildings, waterproof separating layers such as B. bitumen sheeting installed.
Oenology: In oenology , vinometers are used to measure the ethanol content of wines , in which the wine rises more or less depending on the ethanol content. As a non-polar liquid, ethanol reduces the capillary force of wine in a glass tube.
Formula (capillary equation)
The height h of a liquid column is given by:
Where:
- = Surface tension
- θ = contact angle
- ρ = density of the liquid
- g = acceleration due to gravity
- r = radius of the tube
For a water-filled glass tube that is open to the air at sea level (1,013.25 hPa):
- = 0.0728 J / m² at 20 ° C
- θ = 20 ° = 0.35 rad
- ρ = 1000 kg / m³
- g = 9.81 m / s²
the following results for the height of rise:
| Capillary radius | Rise height |
|---|---|
| 1000 mm | 0.014 mm |
| 100 mm | 0.14 mm |
| 10 mm | 1.4 mm |
| 1 mm | 14 mm |
| 0.1 mm | 140 mm |
| 0.01 mm | 1400 mm |
The Washburn equation describes capillary flows in porous materials without considering gravitation .
Capillary barrier
A capillary barrier is a construction that prevents a capillary effect from sucking off liquid. It is used for example:
- in liner ponds , there pond liner is pulled up on the bank to prevent water from being sucked out of the pond by capillary effects in sand or soil. About 5 cm up excess film is covered with stones, kapillarbrechendem fine gravel or concrete grout or by vegetation (water side and outside the pond) or a bank of web camouflaged .
- When sealing the surface of landfills and contaminated sites, capillary barriers are used to drain off surface wastewater. A capillary block (a coarse-grained layer of gravel) is applied and a fine-grained capillary layer (for example sand) over it. When water seeps in, the fine-grained material has a higher water content than the coarser pores in the coarse-grained material. In the fine-grained material, the water is held in the capillary layer by capillary forces, runs down the slope and is drained off at the foot of the slope via drainage pipes (see).
- A horizontal barrier prevents water from rising capillary in masonry, see also rising damp .
- A capillary-breaking layer does this below the foundation level or the floor of a building
- Sometimes a vapor barrier for thermal insulation is also made watertight in order to prevent capillary suction at the same time.
Web links
- Lecture Medical Physics: Surface Tension and Capillarity, University of Veterinary Medicine Vienna ( Memento from March 26, 2012 in the Internet Archive )
Literature and web links
- H. Schubert: Capillarity in porous solid systems . Springer, Berlin 1982, ISBN 3-540-11835-7
- Valentin L. Popov: Contact Mechanics and Friction. A text and application book from nanotribology to numerical simulation . Springer, 2009, 328 p., ISBN 978-3-540-88836-9 .
- Video: YOUNG contact angle and capillarity - how high does water rise in a capillary? . Jakob Günter Lauth (SciFox) 2013, made available by the Technical Information Library (TIB), doi : 10.5446 / 15673 .
Individual evidence
- ↑ proholz.at ( memento from September 23, 2011 in the Internet Archive ).
- ↑ George W. Koch, Stephen C. Sillett, Gregory M. Jennings, Stephen D. Davis: The limits to tree height . Nature 428, 2004, pp. 851-854, doi: 10.1038 / nature02417 .
- ↑ garden ponds. P. 8 ( limited preview in Google Book Search), last accessed in February 2020
- ↑ The capillary barrier. Innovative surface sealing for landfills and contaminated sites . Springer-Verlag 1999.
- ↑ Wolf-Ulrich Henken-Mellies, S. Melchior, B. Steinert: E 2-33 Capillary Barriers in Surface Sealing Systems ; LGA Landesgewerbeanstalt Bayern, Grundbauinstitut, Nuremberg, 2010, (PDF file)