Earth atmosphere

The atmosphere of the earth , and earth's atmosphere (from ancient Greek ἀτμός ATMOS , German , steam ' and σφαῖρα sfaira , German , ball' ) is the gas -like shell of the earth's surface and one of the so-called Erdsphären . It has a high proportion of nitrogen and oxygen and thus oxidizing conditions.
Their vertical structure is due to different temperatures . The weather takes place in the lower 10 kilometers, the troposphere . The higher layers no longer have much influence.
composition
The layers close to the ground up to a height of about 90 km ( Kármán line of space travel) have a fairly uniform composition, which is why one speaks of the homosphere . What is referred to as air consists essentially of:
78.08% nitrogen (N 2 ), 20.95% oxygen (O 2 ) and 0.93% argon, disregarding the changing water vapor content (i.e. in volume percent dry, water vapor-free air) (Ar), plus aerosols and trace gases , including carbon dioxide (CO 2 , currently 0.04%, the most important cause of the greenhouse effect after water vapor ), as well as methane (CH 4 ), ozone (O 3 ), chlorofluorocarbons , sulfur dioxide (SO 2 ) and nitrogen compounds.
For the development of the weather in addition to the power supply by solar radiation and its daily and seasonal variation mainly the content of water vapor responsible. This occurs in the air in varying concentrations from 0 % vol. To approx. 4% vol., See humidity . The regional solar radiation depends on the aerosol content and the transparency of the atmosphere .
The high atmosphere is already a very thin gas into which high-energy components of solar radiation can still penetrate. The molecules are dissociated and partially ionized by short-wave UV light . Furthermore, at altitudes over about 100 km there is also a segregation of the components according to their different molar mass , which is why this section is also called the heterosphere . The proportions of lighter particles such as hydrogen atoms and helium therefore increase with increasing altitude . These two elements gradually escape into space due to thermal factors.
construction


Layering
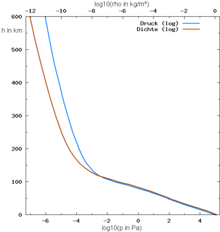
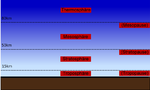
The atmosphere has a mass of about 5.15 · 10 18 kg (5.15 quadrillion tons), that is almost one millionth of the mass of the earth . With regard to its vertical temperature profile, especially its gradient , it consists of several layers:
- Troposphere from the earth's surface to the tropopause at heights between 7 km ( polar regions ) and 17 km ( tropics )
- Stratosphere up to the stratopause at an altitude of 50 km
- Mesosphere up to the mesopause at an altitude of 80 to 85 km
- Thermosphere (see also below, ionosphere)
- Exosphere above.
The troposphere is also referred to as the lower atmosphere , the stratosphere and mesosphere together as the middle atmosphere, and the thermosphere as the upper atmosphere .
Especially in the troposphere - the weather sphere - there is a dynamic within the temperature stratification and the content of water vapor , which is why the respective stratification stability also plays a major role there. In addition to the temperature profile, the air envelope can also be classified according to other aspects:
- According to the aerodynamic state
- Prandtl layer (approx. 0–50 m)
- Ekman layer (approx. 50-1000 m)
- Prandtl layer + Ekman layer = planetary boundary layer = peplosphere
- Free atmosphere (> 1 km)
- According to the degree of mixing
- The homosphere is mixed up in a turbulent manner and extends to homopause at an altitude of about 100 km.
- The heterosphere begins above this. Here the particles separate according to their molar mass, since molecular diffusion dominates.
- According to the radio-physical state of the atmosphere
- Neutrosphere (gases predominantly in a neutral, i.e. non-ionized state)
- Ionosphere (ionized gases, stored in the thermosphere,> 80 km)
- Plasma sphere (complete ionization of all particles,> 1000 km,)
- Magnetosphere
After chemical point of view, also the leave ozonosphere (ozone layer in 16-50 km altitude) and a chemosphere define (20-600 km).
Boundary to space
The transition between exosphere and space is continuous; you cannot draw a sharp upper limit for the atmosphere. In the exosphere (above the exobase at ~ 600 km altitude) the mean free path is so large that particles can escape if they can reach the escape speed . This is made possible for individual hydrogen particles through collisions at average speeds of 3–4 km / s.
By the International Aeronautical Federation is the Homo break (or a height of about 100 km Kármán line ) considered border. This definition is widely recognized internationally, even if it does not have unlimited validity. For example, NASA defines the mesopause (around 80 km) as a limit.
exploration
The lower atmosphere, especially the troposphere , is the research field of meteorology , whereas the middle and upper atmosphere ( stratosphere , mesosphere ) belong to the field of aerology .
Measurements are made close to the ground with the full range of meteorological measuring devices. Radiosondes , meteorological rockets , lidars , radars and weather or environmental satellites are the most important measurement methods in terms of height, especially with regard to height profiles . In the future, high-altitude platforms such as the High Altitude and Long Range Research Aircraft will likely play a greater role.
development
The development of the atmosphere is part of the chemical evolution of the earth and also an important element of climate history . Today it is divided into four main stages of development.
It all began with the formation of the earth around 4.56 billion years ago. Very early on, it possessed a gas envelope presumably consisting of hydrogen (H 2 ) and helium (He), which however was lost again.
The slow cooling of the earth and the resulting volcanism resulted in extensive outgassing from the earth's interior. The atmosphere thus created consisted of about 80% water vapor (H 2 O), 10% carbon dioxide (CO 2 ) and 5 to 7% hydrogen sulfide . These are the products of volcanism that can still be observed today. The high proportion of water vapor can be explained by the fact that the atmosphere at that time was still too warm to be able to form precipitation. So there were no bodies of water on earth. The actual origin of the water is disputed.
After the temperature of the atmosphere fell below the boiling point of water, there was an extremely long continuous rain, after which the oceans had formed and the other atmospheric gases were accordingly enriched relative to the water vapor.
The high levels of UV radiation caused a photochemical breakdown of the water, methane and ammonia molecules, whereby carbon dioxide and nitrogen were relatively enriched. The light gases such as hydrogen or helium evaporated into space. Carbon dioxide was dissolved in large quantities in the oceans and partly consumed by C-autotrophic microorganisms. The inert nitrogen remained unchanged. This was further enriched relatively over time and formed the main component of the atmosphere around 3.4 billion years ago.
The oxygen O 2 plays the main role in the further development of today's atmosphere. Cyanobacteria with oxygenic photosynthesis , as C-autotrophs, led to a further decrease in the carbon dioxide concentration, but mainly formed oxygen (possibly beginning about 3.5 billion years ago). However, the oxygen concentration in the atmosphere initially remained low because the oxygen formed in the oceans was consumed in the oxidation of iron (II) ions and hydrogen sulfide. It was only about two billion years ago that oxygen began to escape into the atmosphere, when the substances that react with oxygen became scarce. A billion years ago, the concentration of oxygen in the atmosphere exceeded three percent, which allowed a first ozone layer to gradually form over the next 400 million years . 500–600 million years ago, the oxygen content rose rapidly due to the first massive appearance of land plants and reached today's level for the first time 350 million years ago. After several strong fluctuations during the Mesozoic Ages , the oxygen content in the air finally reached today's value of 21%.
literature
- Helmut Kraus: The Earth's Atmosphere - An Introduction to Meteorology. Springer, Berlin 2004, ISBN 3-540-20656-6 .
- Kshudiram Saha: The earth's atmosphere - its physics and dynamics . Springer, Berlin 2008, ISBN 978-3-540-78426-5
- Mark Z. Jacobson : Fundamentals of atmospheric modeling. Cambridge University Press, Cambridge 2005, ISBN 0-521-54865-9
- CN Hewitt: Handbook of atmospheric science - principles and applications . Blackwell, Malden (Massachusetts) 2003, ISBN 0-632-05286-4
- Kristian Schlegel: From the rainbow to the polar light - luminous phenomena in the atmosphere . Spectrum, Akademischer Verlag, Heidelberg 2001, ISBN 3-8274-1174-2
- Edmond Murad, Iwan P. Williams: Meteors in the earth's atmosphere - meteoroids and cosmic dust and their interactions with the earth's upper atmosphere . Cambridge University Press, Cambridge 2002, ISBN 0-521-80431-0
Web links
- Structure of the earth's atmosphere - education server - structure
- astronomie.de: Atmosphere - Mesosopharies
- Atmospheric models of the National Space Science Data Center (English)
- The climate information project for schools and the general public
- scinexx.de: Earth's atmosphere extends to the moon February 22, 2019