Calcium chloride
| Crystal structure | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| __ Ca 2+ __ Cl - | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Calcium chloride | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Ratio formula | CaCl 2 | |||||||||||||||||||||
| Brief description |
colorless and odorless, hygroscopic crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass |
|
|||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
|
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| boiling point |
1670 ° C |
|||||||||||||||||||||
| solubility |
good in water (740 g l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Calcium chloride (also calcium chloride ) is a chloride of the alkaline earth metal calcium with the ratio formula CaCl 2 . Calcium is in the +2 oxidation state , chlorine has the −1 oxidation state.
history
Around 1860, the chemists Robert Wilhelm Bunsen and Augustus Matthiessen succeeded in producing the first pure representation of the element calcium by melting electrolysis of calcium chloride.
Occurrence
Calcium chloride occurs naturally in solution in brine .
Calcium chloride containing water forms the rare minerals Sinjarit (dihydrate) and Antarcticit (hexahydrate). The anhydride occurs as a hydrophilite .
Extraction and presentation
Calcium chloride is made from hydrochloric acid and calcium carbonate :
Subsequent heating to 260 ° C provides the anhydrous form.
Technically, calcium chloride is obtained as a waste product in the production of soda by the Solvay process - namely when the ammonia is recovered from the ammonium chloride that is formed:
properties
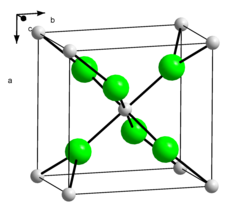
Calcium chloride is a salt . Calcium and chlorine are ionic due to the large difference in electronegativity . The bond thus takes place via electrostatic interactions. Calcium chloride forms colorless crystals that form a distorted rutile structure ( orthorhombic , space group Pnnm (space group no. 58) ).
Calcium chloride forms colorless crystals in its pure form and is highly hygroscopic in an anhydrous state . It easily takes water from the environment, forming a hydrate - complex . Several crystalline hydrates are known. The dihydrate crystallizes orthorhombically in the space group Pbcn (space group no. 60) with the lattice parameters a = 5.893 Å , b = 7.469 Å and c = 12.07 Å. The tetrahydrate is polymorphic . The α-form crystallizes triclinic , space group P 1 (No. 2) , with the lattice parameters a = 6.593 Å, b = 6.367 Å, c = 8.561 Å, α = 97.83 °, β = 93.5 ° and γ = 110.6 °. The β-form has a monoclinic structure with the space group P 2 1 / c (No. 14) , lattice parameters a = 8.923 Å, b = 10.22 Å, c = 12.79 Å and β = 114.7 °. The γ form of the tetrahydrate also crystallizes monoclinically in the space group P 2 1 / c (No. 14) with the lattice parameters a = 6.139 Å, b = 7.667 Å, c = 8.901 Å and β = 111.0 °. The hexahydrate has a trigonal structure, space group P 321 (No. 150) with the lattice parameters a = 7.876 Å and c = 8.561 Å. Corresponding complexes with ammonia are also known (CaCl 2 · n (NH 3 ) with n = 1,2,4,8).
The anhydrous calcium chloride dissolves exothermically in water .
The compound forms stable solvates with methanol and ethanol at room temperature . Solvates of stoichiometry 1: 2, 1: 3, 1: 4, 1: 6 and 1: 8 based on calcium chloride were obtained with methanol and solvates of stoichiometry 1: 2, 1: 3, 1: 4 and 1: 6 with ethanol produced and characterized on calcium chloride.
Reactions
Calcium chloride reacts with water to form a hexahydrate complex and generate a lot of heat ( exothermic , ΔH <0):
The crystals of the hexahydrate dissolve at about 30 ° C in its own water of crystallization . Heating to around 200 ° C releases the bound water again. In contrast to anhydrous calcium chloride, dissolving in water leads to a strong cooling. Both calcium chloride forms are also readily soluble in ethanol .

use
Due to its hygroscopicity, anhydrous calcium chloride is an important drying agent in the laboratory, for example in the desiccator , and in technical chemistry for gases and liquids.
It is also used as road salt ( de-icing agent ) and as a hexahydrate for the production of cold mixtures .
Areas of application in construction are the drying of living spaces , the use as an antifreeze , especially as an antifreeze and setting accelerator in concrete , as well as a dust binder (for example on construction sites and as a filler during blasting work). The use of calcium chloride as a setting accelerator in concrete was banned in Germany in 1963 because of the corrosive effect of chloride on the iron reinforcement .
The filling water of concrete pool calcium chloride is added to adjust the water hardness and to increase according to the Le Chatelier's Principle , the concrete erosion to reduce by dissolving calcium compounds from the concrete.
In addition to anti-freeze, most of the calcium chloride is used to bind sand and dust on unpaved roads. Since it is highly hygroscopic , a concentrated calcium chloride solution applied to the road attracts moisture and suppresses the removal of road dust. The road surface has to be leveled less often and the surface renewed less often.
The food industry uses it as a complexing agent , flavor enhancer and stabilizer (among other things in drinking water treatment ). It is approved in the EU as a food additive with the number E 509 . The daily intake is assumed to be 160–345 mg.
As a firming agent is canned vegetables and sliced fruits added. Also it is used for coagulation of proteins in food production, for example in the production of cheese , tofu or artificial caviar. When making cheese , calcium chloride is sometimes added to milk to improve the properties of the precipitated casein .
It serves as an electrolyte in sports drinks .
Due to its salty taste, it can replace table salt in pickled cucumbers and pickled vegetables .
When brewing calcium chloride is used to determine the mineral content of the brewing water to equalize and affect taste and yeast growth.
Calcium chloride is used to treat the surface of fruit. Apples are treated in the late growth phase in order to avoid the deficiency disease speck and other impairments.
Taking advantage of the exothermic hydration in the reaction with water, calcium chloride is used to heat ready-made beverages.
In molecular biology it is used to produce competent cells . Calcium ions change the permeability of the cell membrane and thus increase the cell's absorption potential for DNA .
In marine aquariums , calcium chloride is used to increase the calcium content.
Individual evidence
- ↑ Entry on E 509: Calcium chloride in the European database for food additives, accessed on June 27, 2020.
- ↑ Entry on CALCIUM CHLORIDE in the CosIng database of the EU Commission, accessed on February 24, 2020.
- ↑ a b c d e f g h Entry on calcium chloride in the GESTIS substance database of the IFA , accessed on February 18, 2017(JavaScript required) .
- ↑ a b c d e Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 0-444-59553-8 , pp. 54 ( limited preview in Google Book search).
- ↑ Data sheet Calcium chloride dihydrate, for molecular biology, ≥99% from Sigma-Aldrich , accessed on May 1, 2017 ( PDF ).
- ↑ Entry on Calcium chloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry on Calcium chloride anhydrous in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ^ AK van Bever, W. Nieuwenkamp: The crystal structure of calcium chloride, CaCl 2 . In: Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie, 1935, 90 , pp. 374–376.
- ^ A. Leclaire, MM Borel: Le dichlorure de calcium dihydrate. In: Acta Crystallographica , B33, 1977, pp. 1608-1610, doi: 10.1107 / S0567740877006645 .
- ↑ A. Leclaire, MM Borel: Liaisons hydrogene et coordination du calcium dans les cristaux de CaCl 2 · 4 H 2 O α. In: Acta Crystallographica , B35, 1979, pp. 585-588, doi: 10.1107 / S0567740879004209 .
- ↑ A. Leclaire, MM Borel: La forme β du dichlorure de calcium tetrahydrate. In: Acta Crystallographica , B34, 1978, pp. 900-902, doi: 10.1107 / S0567740878004288 .
- ↑ A. Lecklaire, MM Borel, JC Monier: La forme γ du dichlorure de calcium tétrahydraté. In: Acta Crystallographica , B36, 1980, pp. 2757-2759, doi: 10.1107 / S0567740880009909 .
- ^ PA Agron, WR Busing: Calcium and strontium dichloride hexahydrates by neutron diffraction. In: Acta Crystallographica , C42, 1986, pp. 141-143, doi: 10.1107 / S0108270186097007 .
- ^ S. Westman, P.-E. Werner, T. Schuler, W. Raldow: X-Ray Investigations of Ammines of Alkaline Earth Metal Halides. I. The Structures of CaCl 2 (NH 3 ) 8 , CaCl 2 (NH 3 ) 2 and the Decomposition Product CaClOH. In: Acta Chemica Scandinavica , 35A, 1981, pp. 467-472, doi: 10.3891 / acta.chem.scand.35a-0467 .
- ↑ Korhammer, K .; Mihaly, J .; Balint, S .; Trif, L .; Vass, A .; Tompos, A .; Talas, E .: Reversible formation of alcohol solvates and their potential use for heat storage in J. Therm. Anal. Calorim. 138 (2019) 11-33, doi : 10.1007 / s10973-019-08090-2 .
- ↑ Jochen Stark: Durability of concrete. Springer-Verlag, 2013, ISBN 978-3-642-35278-2 , p. 263 ( limited preview in Google book search).
- ↑ Dust: Don't Eat It! Control It! . In: Road Management & Engineering Journal . US Roads (TranSafety Inc.). June 1, 1998. Archived from the original on October 29, 2007. Info: The archive link was automatically inserted and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Retrieved August 9, 2006.
- ^ Calcium Chloride SIDS Initial Assessment Profile, UNEP Publications, SIAM 15, Boston, 22-25 October 2002, pp. 13-14.
- ↑ Apple Caviar Technique . In: StarChefs Studio . StarChefs.com. April 2004. Retrieved August 9, 2006.
- ^ "Cork Spot and Bitter Pit of Apples", Richard C. Funt and Michael A. Ellis, Ohioline.osu.edu/factsheet/plpath-fru-01




