Calcium bromide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Ca 2+ __ Br - | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Calcium bromide | ||||||||||||||||||
| other names |
Calcium dibromide |
||||||||||||||||||
| Ratio formula | CaBr 2 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 199.88 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
730 ° C |
||||||||||||||||||
| boiling point |
806-812 ° C |
||||||||||||||||||
| solubility |
good in water (1420 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Calcium is the calcium salt of hydrobromic acid ( bromides ). It is a colorless solid that slowly turns yellow in air.
Extraction and presentation
Calcium bromide can be obtained by reacting calcium carbonate , calcium oxide or calcium hydroxide with hydrogen bromide .
An alternative is the reaction of the calcium salts with bromine and a reducing agent such as formic acid or formaldehyde .
properties
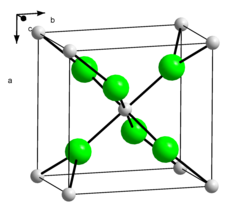
Calcium bromide, like calcium chloride and strontium chloride , crystallizes in the calcium chloride structure , which is similar to the rutile structure . In the gaseous state it forms linear molecules. A crystalline hexahydrate is also known which crystallizes trigonally in the space group P 321 (space group no. 150) with the lattice parameters a = 8.164 Å and c = 4.016 Å.
The compound slowly turns yellow in air as elemental bromine is formed. Calcium bromide is soluble in water and methanol , but only slightly soluble in ether and chloroform .
use
Calcium bromide is used for the production of photo plates , as a medicine , in wood preservatives and in flame retardants .
Individual evidence
- ↑ Calcium bromide data sheet from Sigma-Aldrich , accessed on March 15, 2011 ( PDF ).
- ↑ a b c d data sheet calcium bromide from AlfaAesar, accessed on June 9, 2009 ( PDF )(JavaScript required) .
- ↑ a b c d e Yoffe, D .; Frim, R .; Ukeles, SD; Dagani, MJ; Barda, HJ; Benya, TJ; Sanders, DC: Bromine Compounds , in: Ullmanns Enzyklopädie der Technischen Chemie , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2013; doi : 10.1002 / 14356007.a04_405.pub2 .
- ↑ a b Entry on calcium bromide in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1241.
- ↑ A. Leclaire, MM Borel: Le dichlorure et le dibromure de calcium hexahydrates. In: Acta Crystallographica , B33, 1977, pp. 2938-2940, doi: 10.1107 / S0567740877009881 .
- ↑ Entry on calcium bromide. In: Römpp Online . Georg Thieme Verlag, accessed on January 3, 2015.


