Methanol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Methanol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | CH 4 O | ||||||||||||||||||
| Brief description |
colorless liquid with a pleasant to pungent odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 32.04 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.79 g cm −3 |
||||||||||||||||||
| Melting point |
−98 ° C |
||||||||||||||||||
| boiling point |
65 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| pK s value |
16 |
||||||||||||||||||
| solubility |
miscible with water, ethanol and diethyl ether |
||||||||||||||||||
| Refractive index |
1.3288 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−239.2 kJ / mol (liquid) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Methanol ( IUPAC ), also methyl alcohol (obsolete wood spirit or wood alcohol ), is an organic-chemical compound with the empirical formula CH 4 O ( semi-structural formula : CH 3 OH) and the simplest representative from the group of alcohols . Under normal conditions , methanol is a clear, colorless, flammable and highly volatile liquid with an alcoholic odor. It mixes with many organic solvents and with water in all proportions.
With 60 million tons of annual production (as of 2012), methanol is one of the most widely produced organic chemicals. The technical production of methanol is mainly catalytic from carbon monoxide and hydrogen . In the chemical industry, it is used in particular as a starting material in the production of formaldehyde , formic acid and acetic acid .
In addition to their material use, methanol and its derivatives are also used as an energy source. Methanol to Gasoline technology turns methanol into fuel. Methanol is also required in the synthesis of biodiesel and the anti-knock agent MTBE . With the help of fuel cells , it can deliver electrical energy. It is also being discussed as a cheap, high-density long-term storage system for solar and wind energy.
In nature, methanol occurs in cotton plants , fruits and grasses and as a metabolic product of bacteria . In beer brewing , winemaking or the production of spirits , it is released in small quantities as a by-product, mainly through the breakdown of pectins. The breakdown products of methanol, especially formaldehyde , are toxic. Therefore, the consumption of methanol can lead to blindness and, in higher doses, also to death.
history
The ancient Egyptians obtained methanol by pyrolysis of wood (wood spirit) and embalmed their dead with a mixture of substances based on it. The Irish chemist Robert Boyle obtained pure methanol from boxwood for the first time in 1661 using the process of dry distillation . In 1834 the French chemists Jean-Baptiste Dumas and Eugène-Melchior Péligot clarified the composition of this water-clear liquid and also gave it its name "methylene", derived from ancient Greek méthy ( ancient Greek μέθυ ) for intoxicating drink or wine and hylé ( ancient Greek ὕλη ) for Wood composites.
The first synthesis of methanol was achieved by Marcelin Berthelot in 1858 by saponification of methyl chloride .
In 1930, the United States was still extracting about 50% of the methanol produced by dry distillation of wood. For this, wood was heated to around 500 ° C in iron containers. Charcoal remained as a solid residue , the gaseous products were drawn off and partially condensed. In addition to methanol, the resulting aqueous distillate mainly contained acetone , acetic acid and methyl acetate . The separation of these components and the final drying required several neutralization - distillation - and drying steps . The yield of methanol in the dry distillation was about 10% of the mass used.
The BASF received in 1913 a patent for a process for methanol production from kohlestämmigem synthesis gas . Matthias Pier , Alwin Mittasch and Fritz Winkler developed the process and used it for the first large-scale production of methanol, which began in 1923 in the Merseburg ammonia plant of the Leuna -Werke. The process used an oxidic zinc - chromium - catalyst at a pressure of 250 atm to 300 atm. The temperatures were between 360 ° C and 380 ° C and the ratio of carbon monoxide to hydrogen was 1 to 2.2.
The scientists involved recognized early on that copper- based catalysts were much more active. However, these were very sensitive to the sulfur compounds contained in the synthesis gas . The further development of methanol synthesis was linked to advances in coal gasification technology and gas cleaning processes . After it became possible to limit the gases to a sulfur content of less than 0.1 ppm on an industrial scale , in 1966 the ICI company developed the first low-pressure synthesis based on a copper oxide-zinc oxide-aluminum oxide catalyst.
Occurrence
After methane, methanol is the second most common organic gas in the earth's atmosphere and occurs in concentrations of 0.1 to 10 ppb . It is a significant atmospheric source of formaldehyde and carbon monoxide . Most of the methanol in the atmosphere is emitted by plants. In wetlands, methanol emissions of 268 micrograms per square meter and hour were found, while values between 100 and 500 micrograms per square meter and hour were observed on grass and pastures. The methanol is carried out by release pectin methyl esterase (PME) of pectin (partially esterified with methanol poly-galacturonic acid ), such as in response to attack by predators. The total amount of methanol released by plants is estimated to be over 100 million tons per year.
Methyl esters and ethers , in which methanol is chemically bound, are found in many fruits (methyl esters) and in lignin , a component of the plant cell wall ( phenyl methyl ether ). The methyl phenyl ether groups of the coniferyl and sinapyl alcohol units present in the lignin split with the absorption of water in methanol and a phenolic residue.
Methanol is periodically by enzymatic hydrolysis of the galacturonic acid methylester in the maceration released. In order to keep the content of methanol in the end product, which is undesirable because of the toxicity of methanol, as low as possible, attempts are made to minimize the release of methanol by suitable methods. The pectolytic enzyme activity can be minimized by adding acid. Furthermore, the sulfur dioxide content and the temperature of the mash have an influence on the enzymatic activity. By briefly heating the mash to up to 90 ° C and cooling it down quickly, the methanol content can be reduced by 40% to 90%. The methanol content in the spirit can also be kept low by suitable procedural steps during the distillation, for example by condensation of highly volatile components. Alcoholic beverages sometimes contain considerable amounts of methanol. In an investigation of various fruit juices and alcoholic beverages, the Baden-Württemberg Investigation Office found peak values of up to 4.7 g · l −1 methanol in spirits and up to 0.2 g · l −1 in wines and fruit juices .
Tobacco partly contains lignin-containing components, the phenyl methyl ethers of which are split pyrolytically and are responsible for the occurrence of methanol in tobacco smoke. Smoking smoke releases methanol according to the same principle . During the digestion of aspartame , a methyl ester of the dipeptide of the α- amino acids L - aspartic acid and L - phenylalanine , methanol is split off. However, when consuming normal amounts of foods sweetened with aspartame, no toxicologically critical values are reached with regard to methanol.
Methanol is common in interstellar space , although the mechanism of formation has not been clarified. In 2006, astronomers succeeded in observing a large methanol cloud with the MERLIN radio telescope at the Jodrell Bank radio observatory. With the sensitive instruments of the Spitzer Space Telescope , it was possible to detect methanol in protoplanetary disks around young stars.
Manufacturing
Methanol is a basic organic chemical and a large-scale, large-scale alcohol. In 2008, global methanol consumption was 45 million tons. The largest exporters of methanol in 2006 were the Caribbean countries such as Trinidad and Tobago with 7.541 million tons, Chile and Argentina with 3.566 million tons and the countries on the Persian Gulf with 5.656 million tons. The largest importers were the United States with 7.112 million tons, Western Europe with 8.062 million tons, Taiwan and South Korea with a total of 2.361 million tons and Japan with 1.039 million tons.
The technical production of methanol takes place exclusively in catalytic processes from synthesis gas , a mixture of carbon monoxide and hydrogen in a ratio of about 1: 2. These procedures are divided into three areas according to reaction pressures. The high-pressure process initially developed worked due to the low catalyst activity and the volume contraction of the reaction at pressures of 250 to 350 bar and temperatures of 360 to 380 ° C. The medium pressure process works at 100 to 250 bar and 220 to 300 ° C, the low pressure process at 50 to 100 bar and 200 to 300 ° C. Each process works with specific catalysts and mass ratios of carbon monoxide to hydrogen.
The synthesis gas required for the synthesis of methanol can be produced from fossil raw materials such as coal, lignite, petroleum fractions and peat. When using renewable raw materials such as wood, biogas or other biomass, the product is also referred to as biomethanol. Furthermore, garbage or sewage sludge can also be used to produce synthesis gas.
The steam reforming and the partial oxidation of natural gas, the largest economically usable according to current estimates hydrocarbon source are, besides the coal the main supplier of syngas. In North America and Europe, natural gas is mostly used as a raw material; in China and South Africa , synthesis gas production is based on coal or lignite. In 2005, China produced 5.4 million tons of methanol, of which 65% or 3.5 million tons was based on coal.
The following equations can be formulated for the formation of methanol from synthesis gas:
and
Because of the economic advantages at low synthesis pressures and temperatures, methanol is largely produced using the low-pressure process. Dimethyl ether , methyl formate and ethanol are formed as by-products and can be distilled off. The medium pressure processes compensate for the economic disadvantage of the higher pressure with higher yields . The letterpress process is no longer carried out today.
China is the largest producer and consumer of methanol today. The Chinese production capacity alone is expected to exceed 60 million tons per year in the next few years. While most of the methanol is currently used in the chemical sector, its use in the fuel sector has shown the highest growth rates. In 2008, China used around three million tons of methanol as a blending component for the production of fuel blends . The development of suitable engines and other engine components that are compatible with methanol pose problems for widespread introduction and higher proportions of methanol in fuel. In 2000, around two million tons were produced in Germany, of which around 1.4 million tons were made from residual oils .
properties
| Physical Properties | |
| Speed of sound | 1123 m s −1 (25 ° C) |
| Surface tension | 0.0226 Nm −1 (20 ° C against air) |
| Dynamic viscosity | 0.544 10 −3 Pa s (25 ° C) |
| Dielectric constant | 33.8 = (25 ° C) |
| Refractive index | 1.326 (25 ° C, Na D line) |
| Isothermal compressibility | 12 · 10 −5 bar −1 (20 ° C) |
| Heat capacity | 81.08 J mol −1 K −1 (25 ° C) |
| Auto-ignition temperature | 470 ° C |
| Critical temperature | 512.5 K |
| Critical pressure | 8.084 MPa |
| Triple point | 175.5 K |
| Magnetic susceptibility | 5.3 · 10 −7 cm³ · g −1 |
| viscosity | 0.808 mPas (0 ° C) 0.690 mPas (10 ° C) 0.593 mPas (20 ° C) 0.449 mPas (40 ° C) 0.349 mPas (60 ° C) |
| Standard enthalpy of formation | −238 kJ / mol |
| Standard enthalpy of vaporization | +37.4 kJ / mol |
| Standard molar entropy | 127.2 J / (mol K) |
| Standard enthalpy of combustion | −726 kJ / mol |
| Van der Waals equation | a = 964.9 l 2 kPa / mol 2 b = 0.06702 l / mol |
Alcohols that are formally derived from alkanes are referred to as alkanols . Methanol is the simplest alcohol and forms the first member of the homologous series of alkanols. Previously, many alcohols were referred to as carbinols based on a proposal by Hermann Kolbe as derivatives of methanol - derived from carbinol . Since 1957, the IUPAC has recommended that this nomenclature no longer be used.
Physical Properties
Under normal conditions, methanol is a colorless, easily mobile liquid. The boiling point is 65 ° C. Methanol solidifies below −98 ° C in the form of colorless crystals. According to Antoine, the vapor pressure function results from log 10 ( P ) = A - ( B / ( T + C )) ( P in bar, T in K) with A = 5.20409, B = 1581.341 and C = −33 , 5 in the temperature range from 288 to 357 K.
Due to the polarity of the hydroxyl group , hydrogen bonds form between the methanol molecules . While the melting point corresponds almost exactly to that of methyl chloride , the formation of hydrogen bonds in the liquid state leads to a relatively high boiling point compared to the methyl halides . The dissociation energy of the hydrogen bond is about 20 kJ / mol.
Methanol forms azeotropes with a large number of organic compounds such as acetonitrile , benzene , chloroform , cyclopentane , methyl methacrylate and tetrahydrofuran . Methanol mixes with water with volume contraction . With a volume fraction of 55% to 60% methanol before mixing, a mixing volume of 96.36% is obtained.
Methanol crystallizes in the orthorhombic crystal system with the lattice parameters a = 643 pm, b = 724 pm and c = 467 pm. The structure can be described as a chain polymer bound via hydrogen bonds. With further cooling, a phase transition takes place by folding the polymer chain into a monoclinic crystal system .
Molecular Properties
The methanol molecule consists of one carbon , one oxygen and four hydrogen atoms . The molecule has a methyl group with trigonal symmetry and a hydroxyl group as structural units . The data on the molecular geometry are shown in the sketch. The bond angle between the carbon, oxygen and hydrogen atoms is 108.9 ° and is slightly contracted compared to the tetrahedron angle of 109.47 °. The bond length between the oxygen and hydrogen atoms is 96 pm and is therefore smaller than the carbon-hydrogen bond length of the methyl group, which is 110 pm (1.10 Å), due to the greater electronegativity of oxygen.
The rotation inhibition of the carbon-oxygen single bond was determined to be 4.48 kJ / mol and is therefore only a third of the two methyl groups, for example in ethane .
Chemical properties
Due to the polar hydroxyl group, methanol can be mixed with water in any ratio. The similarity to water can be seen in the dissolving power of some mineral salts such as calcium chloride and copper sulfate in methanol. It is also readily soluble in diethyl ether , hydrocarbons and many other organic solvents with the exclusion of water. In some solvents, even small amounts of water can cause separation. Methanol is not very soluble in vegetable fats and oils .
The pK s value of methanol is 16, methanol reacts acidic in aqueous solution. Methanol can be deprotonated to methanolate with strong bases . Methanol can be protonated with strong acids such as sulfuric acid .
Methanol burns with a slightly blue, almost invisible flame to carbon dioxide and water. The flash point is 9 ° C. Methanol vapors form explosive mixtures with air in the range of 6% to 50% . Methanol reacts with alkali and alkaline earth metals to form hydrogen and the methanolates. It reacts easily with many oxidizing agents such as barium perchlorate , bromine or hydrogen peroxide . Various plastics, paints and rubber are attacked by methanol.
Methanol reacts with carboxylic acids in acid or base catalysis with the release of water to form methyl esters ; With carboxylic acid esters , transesterification is possible with the release and removal of the other alcohol component from the reaction mixture or in excess of methanol.
Methanol can be catalytically oxidized to formaldehyde . Methanol reacts with aldehydes and ketones in the presence of acidic catalysts to form hemiacetals or dimethyl acetals , which can be used as protective groups in organic chemistry.
use
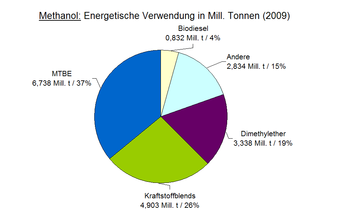
Methanol is used, among other things, as a starting material in the chemical industry or as an energy supplier. The material recycling as a chemical raw material requires a particularly pure product. Raw methanol can be burned as an energy source in stationary plants. A mixture of pure methanol and water also provides the chemical energy to operate fuel cells, which convert them into electrical energy. The use as fuel, so-called fuel-methanol , is being intensively investigated. It can be added to conventional motor fuels or pure methanol can be used. Methanol is used as a polar solvent . In the Rectisol process , it is used to separate acidic components such as carbon dioxide or carbonyl sulfide from gas streams. In the period from 2005 to 2009, the total amount of material methanol used increased by around 6%, while the energy use showed an increase rate of 55%.
Methanol as a chemical raw material
Methanol is an important starting material for syntheses in the chemical industry. Quantitatively, of great importance are the primary derivatives of formaldehyde, acetic acid, MTBE, methyl methacrylate , methyl chloride and methyl amines . These are processed into a number of secondary and tertiary derivatives. Well-known examples are vinyl acetate , acetic anhydride , phenol-formaldehyde resins and melamine resins .
formaldehyde
Most of the methanol processed into formaldehyde is converted by oxidation with oxygen on silver catalysts or in the Formox process on iron oxide / molybdenum oxide / vanadium oxide catalysts at 400 ° C.
The formaldehyde market in North America shrank by around 15% between 2006 and 2010, largely due to the decline in demand in the furniture and construction industries. The market volume in North America was around 4 million tons in 2010. Formaldehyde is mainly used in the manufacture of urea , phenol and melamine formaldehyde resins , the largest consumers of which are the construction , automotive and wood industries . Formaldehyde resins are used in the manufacture of wood products, for example as binders for hardboard and chipboard . Fast growing markets are the manufacture of polyoxymethylene , methylene diisocyanate and 1,4-butanediol . In 2005, China was the world's largest formaldehyde producer with a capacity of 11 million tons.
acetic acid
Methanol is used for the production of acetic acid by reaction with carbon monoxide according to the Monsanto process and for acetic anhydride production via methyl acetate according to the Tennessee-Eastman acetic anhydride process . The catalytically active species is the anionic rhodium complex cis- [Rh (CO) 2 I 2 ] - with hydrogen iodide as co-catalyst .
In the catalytic cycle, methanol first reacts with hydriodic acid to form methyl iodide , which adds oxidatively to the rhodium complex. Carbon monoxide inserts into the metal-methyl bond to form a formyl complex. This is eliminated from the complex as an acid halide . The acid iodide reacts with water again to form hydriodic acid and acetic acid.
In the production of acetic anhydride , part of the product is converted into methyl acetate with methanol and fed back into the process. The acetic anhydride is obtained entirely on the basis of synthesis gas.
Another product derived from this synthesis is vinyl acetate . By hydrocarbonylating a mixture of acetic anhydride and methyl acetate in the presence of homogeneous rhodium catalysts , ethylidene diacetate is formed at temperatures around 150 ° C. and a pressure of about 40 bar to 70 bar , which can split into vinyl acetate and acetic acid at an elevated temperature with acid catalysis.
Other derivative products
Methyl methacrylate , the monomer of polymethyl methacrylate , is produced by hydrolysis and subsequent esterification of the 2-methylpropenenitrile formed from acetone cyanohydrin with sulfuric acid in the presence of methanol.
Methanol can be dehydrogenated to methyl formate with the help of doped copper catalysts . After the hydrogen produced has been separated off, the methyl formate is first washed out in cold methanol and then separated by distillation.
A number of secondary products can be produced by esterification. Chloromethane can be produced selectively by conversion with inexpensive hydrochloric acid. Fatty acid methyl esters can be made by conventional transesterification processes. Dimethyl terephthalate is obtained by a two-stage oxidation of p- xylene with an intermediate esterification step .
The reaction of methanol with ammonia using aluminum silicates as a catalyst produces mixtures of methylamines , a precursor for dyes, medicines and pesticides.
By converting methanol to zeolites of the ZSM-5 type in the methanol-to-olefins process, short-chain olefins such as ethylene , propylene and butenes can be produced , which were previously usually obtained by steam cracking light naphtha . In the first step, dimethyl ether is formed, which further reacts to ethene with elimination of water .
The selectivity to aromatic products can be changed by varying the reaction conditions (Methanol to Aromatics, MtA) .
Methanol in the energy sector
Methanol can serve as a source of energy in a number of ways. It can be used as a raw material for chemical conversion into other fuels. Furthermore, methanol can be used as a 15% mixture with gasoline or directly as pure methanol (M100), the energy density of which is around 52% in relation to motor gasoline . Pure methanol can serve as a hydrogen supplier for fuel cells, or it can be used directly in direct methanol fuel cells (i.e. without the intermediate product hydrogen) to provide electrical energy. In combination with the catalytic generation of the energy storage material, a closed or open cycle can be set up to solve the energy buffer problem of alternative energy sources. Several manufacturing variants involving electrical or photonic reactions are already in use and are currently being actively developed further towards greater efficiency. During the Second World War, mixtures containing methanol were used as fuel for rocket and aircraft engines ( MW-50 ). Thus C-substance , a mixture of methanol, hydrazine , water and Kaliumtetracyanidocuprat (I) (K 3 [Cu (CN) 4 ]), together with T-fabric , a high concentration of hydrogen peroxide , as a self-igniting, hypergolic used fuel.
Methanol as fuel
| Methanol fuel | |
|---|---|
| other names |
M100, Methol, Spritol, Methyloxyhydrat, Methynol, Pyroholzether, Spiritol, Holzin, wood alcohol, wood spirit, carbinol, wood spirit, carbinol, methyl alcohol |
| Brief description | Petrol for adapted engines |
| Characteristic components |
Methanol |
| CAS number |
67-56-1 |
| properties | |
| Physical state | liquid |
| density |
0.79 kg / l |
| calorific value |
15.7 MJ l −1 = 19.9 MJ kg −1 |
| Calorific value |
17.9 MJ l −1 = 22.7 MJ kg −1 |
| Octane number |
106 RON |
| Flash point |
9 ° C |
| Ignition temperature | 440 ° C |
| Explosive limit | 6–50% by volume |
| Temperature class | T2 |
| safety instructions | |
| UN number | 1230 |
| Hazard number | 336 |
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |
Methanol can be used either directly as a fuel or as a fuel additive in a variety of ways. Several possibilities are known today for use in Otto and diesel internal combustion engines . According to the European standard for petrol EN 228 , maximum admixtures of 3 percent by volume to the fuel are permitted with the addition of stabilizers . Such small admixtures can be withstood by today's gasoline engines without adjustments. For cost reasons, Germany has not yet made use of these possibilities.
Furthermore, methanol can be used as an admixture in higher concentrations to gasoline or as an almost pure methanol fuel. In Germany, the Federal Ministry of Education and Research sponsored a large-scale test in the 1980s with an M15 fuel, consisting of 15% methanol and 85% gasoline, and with an M85 fuel with corresponding ratios; it tested these extensively with over 1000 vehicles from all German car manufacturers with the support of the mineral oil industry and numerous research institutes . The vehicles have been adapted in terms of materials and mixtures for operation with these fuels. The United States, Japan, China, New Zealand, and South Africa conducted similar experiments. This program also tested a methanol-diesel mixed fuel with 20% methanol in cars.
Commercial vehicle diesel engines have been modified to use pure methanol (M100). Because of the low cetane number of methanol, it is not possible to operate the engine as a compression ignition . The testers therefore used additional ignition aids in the form of diesel pilot injection or spark ignition or glow ignition . The tanker Lindanger is the type ship for seven product tankers whose dual-fuel two-stroke engines are powered by methanol. The dual-fuel main engines of the type B&W 6G50ME-9.3 LGIB with a nominal output of 10,320 kW at 100 rpm are driven with methanol and MGO as ignition oil . They were developed by MAN B&W in Copenhagen and built by the engine and mechanical engineering department of Hyundai Heavy Industries.
A two-fuel diesel-methanol operation is also possible. The methanol glow igniter engine developed by Franz Pischinger has good emission values with low consumption.
In engines adapted for pure methanol M100 and M85, compared to gasoline engines, up to 10% higher engine output and about 15% better thermal efficiency can be achieved, thus lower energy consumption . As a liquid fuel, methanol is particularly suitable for the transport sector because of its ease of use compared to gaseous fuels, for road, water and rail transport and, with restrictions, for aviation.
While there are no longer any advantages for the limited emissions for hydrocarbons, carbon monoxide and nitrogen oxides with the catalytic converter technology commonly used in gasoline engines today, there are minor advantages for the non-limited emissions. For example, methanol does not emit aromatics such as benzene , toluene and lower polycyclic aromatic hydrocarbons and has a low ozone formation potential . On the other hand, the disadvantage is the increased formaldehyde emission, the level of all emission components listed here being very low because of the catalyst. With diesel concepts, there are largely no sulfur emissions and soot formation . Methanol has almost 50% of the calorific value of diesel and gasoline.
The toxicity of methanol is disadvantageous and requires precautionary measures when refueling and when working on the vehicle. Since methanol is biodegradable , there is little risk to the environment in the event of an accident.
In US motorsport in the 1960s, the American formula racing series ( CART , Indy Car ) replaced petrol, which could not be extinguished with water, with methanol after serious fire accidents in the Indianapolis 500 . A disadvantage is that burning pure methanol is barely visible. After the refueling process in the race, water is always sprayed over the filler neck to wash away any leaked methanol before it ignites on hot parts. Like ethanol, methanol is particularly suitable for supercharged engines . Dragsters with eight-liter supercharged V8 engines of the Top Methanol class achieve outputs of over 3500 hp.
In model making, methanol with added nitromethane is used in glow igniter engines. Its use is falling sharply because model building fuels are expensive and modern electric motors with lithium-ion batteries are becoming cheaper and quieter.
Methanol as an energy supplier for fuel cells
There are two ways in which methanol can be used to obtain electrical energy from fuel cells: Either the methanol serves as a hydrogen supplier for a hydrogen fuel cell, or it is converted directly in the fuel cell. In order to supply hydrogen fuel cells, the methanol must first be converted into hydrogen and carbon dioxide CO 2 by adding energy . A methanol reformer is used for this step . Then the hydrogen is separated from the CO 2 (and any CO) and fed to the fuel cell, where it is converted. As an alternative to the combination of reformer and H 2 cell, suitable fuel cells, the direct methanol fuel cells , can use the methanol-water mixture directly, i.e. without prior conversion into hydrogen. In terms of the functional principle, this is the simpler variant. It is also preferred for small consumers such as refrigerators or televisions when camping or measuring equipment. According to the company, over 40,000 such direct methanol fuel cells have already been sold.
The reactions that take place are:
Anode reaction:
Cathode reaction:
Overall reaction:
This type of cell uses a proton exchange membrane as the electrolyte . The methanol-water mixture is fed to the anode and the methanol is oxidized there, producing carbon dioxide as the exhaust gas . At the cathode , the hydrogen ions react with atmospheric oxygen to form water. One problem with direct methanol fuel cells is that the membrane is permeable to methanol, which in turn reduces efficiency.
Methanol derivatives as fuel
The primary derivatives of methanol are already used in many ways as fuel or fuel additive. The use of the octane booster MTBE, which was approved in the United States in 1979 by the EPA in concentrations between 2 and 5%, is known. The derivative dimethyl ether (DME) is used as a diesel substitute fuel. Methanol is used for the transesterification of vegetable oil and the production of biodiesel. Advantages of the derivatives include their freedom from sulfur and aromatics. The energy density is higher than that of pure methanol.
Biodiesel
In biodiesel production , methanol is used to transesterify vegetable oils. For example, rapeseed oil is transesterified with methanol under base catalysis. The methanol is added beyond the stoichiometric ratio of glycerol ester to alcohol in order to shift the reaction to the side of the methyl ester . Glycerine is a by-product. After the reaction has ended, the phases are separated and the biodiesel is washed and distilled for processing. Modern biodiesel plants have a capacity of around 200,000 tons per year; the total installed capacity in Germany in 2006 was 3,840,500 t.
Methanol to Gasoline
In the methanol-to-gasoline process, methanol is used to produce high-octane carburetor fuels. By conversion of the zeolite - catalysts of the type ZSM-5 is the intermediate product dimethyl ether, a hydrocarbon mixture formed. The reaction takes place in the first step via the dehydration of the dimethyl ether to ethene and other light olefins, which can oligomerize and cyclize in further steps to products with five or more carbon atoms . The reaction mechanism is complex and has been the subject of intensive research to this day.
The residence times are longer and temperatures are higher than with the related MtO and MtA processes. The petrol obtained is sulfur-free and has a low benzene content. The process can be carried out in a fixed bed or fluid bed method. The fluid bed process has advantages through continuous catalyst regeneration , which allows lower pressures. Total built a pilot plant with a daily production capacity of 1,700 tons of fuel in New Zealand. Rheinbraun operated another pilot plant for a long time in Berrenrath, North Rhine-Westphalia . It was built jointly by Uhde and Lurgi .
MTBE
By acid-catalyzed conversion of methanol with isobutene is methyl tertiary butyl ether (MTBE), an octane enhancer prepared. The oxygen content of MTBE results in better fuel combustion in carburettor engines. The air improvement achieved in this way was the main reason why the use of oxygenates, a group of chemicals that increase the oxygen content of gasoline , was prescribed in the United States' Clean Air Act (CAA) of 1992. The refineries achieved the target of 2.7% by weight oxygen in fuel specified in the ordinance , primarily through the use of MTBE.
After MTBE was detected in groundwater, in 2003 California and other US states banned the use of MTBE as an octane booster, as concentrations of around 40 µg MTBE per liter of water impair the quality of drinking water. In Europe and Germany, the use of MTBE was increased by Directive 85/535 / EEC and later by the Fuel Quality Directive 98/70 / EC, according to which an admixture of up to 15% by volume is permitted. In Germany and the EU, investigations have not found any direct health or environmental hazard from MTBE, and a ban was not considered.
Dimethyl ether
The gas dimethyl ether (DME), which is easy to liquefy, can be produced by catalytic dehydrogenation of methanol in the presence of silica-alumina catalysts. DME is seen by some companies as a promising fuel in diesel engines and gas turbines. The cetane number of DME is 55 and thus higher than that of conventional diesel. The combustion is relatively clean and only leads to low emissions of particles, nitrogen oxides and carbon monoxide. In the course of the European BioDME project, it is investigated whether DME produced on the basis of lignocellulose can be produced on an industrial scale.
Other uses
Methanol is also used in many areas. It is used as a solvent and antifreeze . In heat pipes in the medium temperature range up to 500 K, methanol is used as the transfer fluid . It is also used to clean the sensors of digital SLR cameras , as it does not leave any streaks and evaporates without leaving any residue. Mono- and perdeuterated methanol are used as solvents in nuclear magnetic resonance spectroscopy . In sewage treatment plants, methanol is added to the wastewater to support denitrification , the conversion of nitrate into gaseous nitrogen. The bacterial metabolic processes require methanol as an additional energy supplier. In waste processing, methanol is used for the solvolytic recycling of polyethylene terephthalate . In the process, ethylene glycol and dimethyl terephthalate are recovered. Methanol is used to separate polystyrene and chloroprene rubber from polymer mixtures, for example to encapsulate other polymers such as butadiene rubber .
The use of methanol to transport coal in methanol-coal slurries has been studied intensively. In this process, the coal-methanol slurry can be burned directly or the methanol can be separated off by distillation and pumped back to the place where the coal is extracted via pipelines. Methanol is used as an extraction agent in the chemical and oil industries, for example to separate aromatic and paraffinic hydrocarbons.
Biological importance
Methanol as a substrate in anaerobic metabolism
Methanol is not only broken down into carbon dioxide for the purpose of generating energy, but can also serve as a carbon source for the construction of cell components. This is particularly the case for anaerobic methanotrophs that assimilate C 1 compounds . As a rule, methanol is first oxidized to formaldehyde and can be built up to carbohydrates either in the Wood-Ljungdahl route , the serine route or in the ribulose monophosphate route .
Methanol as an intermediate product in the aerobic degradation of methane
Methanol is formed as an intermediate product of the metabolism of methanotrophic bacteria from the oxidation of methane. Methylotrophic bacteria ( Methylophilaceae ) and yeasts, such as baker's yeast , also oxidize other C 1 compounds such as methanol and formaldehyde to produce energy. The degradation takes place in aerobic environments near methane deposits.
The aerobic biodegradation of methane takes place via the stages of methanol, formaldehyde and formate to form carbon dioxide (CO 2 ).
The following applies to the overall reaction:
The oxidation of methane to methanol is catalyzed by the enzyme methane monooxygenase with consumption of oxygen and nicotinamide adenine dinucleotide (NAD (P) H). The further oxidation of the resulting methanol to formaldehyde takes place in different ways depending on the species. Gram-negative bacteria oxidize methanol via a soluble methanol dehydrogenase in the periplasmic space with pyrroloquinoline quinone (PQQ) as a coenzyme . Gram-positive, methanotrophic bacteria such as Bacilli and actinomycetes use a cytosolic NAD (P) H-dependent dehydrogenase. In contrast, yeasts oxidize methanol in the peroxisomes , which is catalyzed by an FAD -dependent alcohol oxidase. The electrons are transferred to oxygen, so that hydrogen peroxide is produced.
Various metabolic pathways are known for the oxidation of formaldehyde. Formaldehyde is very reactive and is bound , for example, as an adduct to tetrahydrofolic acid or tetrahydromethanopterin , alternatively to glutathione .
Single cell proteins
A method for producing unicellular proteins (single cell protein) based on methanol have been extensively studied. For example, bacteria of the type Methylophilus methylotropha are fermented in airlift reactors , using ammonia as a nitrogen source . In this way, protein-rich products are obtained whose amino acid composition is similar to that of fish meal . The use of single-cell proteins based on methanol for feed purposes is toxicologically and nutritionally harmless. After appropriate processing, the proteins can be used as food. An ICI process has already been implemented on an industrial scale, but the products could not be marketed compared to inexpensive soy and fish meal products. The advantage of using methanol over other carbon sources is, in addition to its miscibility with water, the lower oxygen requirement and the lower heat generation during fermentation.
Deuterated methanol
There are three different deuterated variants of methanol:
- Methanol-d1 , also MeOD , in which only the hydrogen atom of the hydroxyl group has been exchanged for deuterium
- Methanol-d3 , in which the hydrogen atoms of the methyl group have been replaced
- Methanol-d4 in which all hydrogen atoms have been exchanged
Methanol-d4 (perdeuteromethanol) is used as a solvent in nuclear magnetic resonance spectroscopy (NMR).
toxicology
Methanol is easily absorbed by inhalation , ingestion, or skin contact. It is quickly distributed in the body through body fluid. Small amounts are excreted unchanged via the lungs and kidneys.
Metabolism and toxic effects are the same as those observed for ethylene glycol . Raw material methanol is the replaced only by low toxicity ( toxicity ). Its breakdown products ( metabolites ) are toxic , such as the formaldehyde formed by ADH ( alcohol dehydrogenase ) (see figure on the right) and the formic acid produced from it . The latter in particular leads to the development of metabolic acidosis after a latency period of 6 to 30 hours, which is often without symptoms . Formic acid is only broken down very slowly by the human metabolism and thus accumulates in the body during the comparatively rapid breakdown of methanol. The toxicity of formaldehyde is controversial in methanol poisoning. Due to the catalytic action of the enzyme aldehyde dehydrogenase, it is further broken down into formic acid very quickly, so that there is no accumulation of formaldehyde in the body. Doses from 0.1 g of methanol per kg of body weight are dangerous, over 1 g per kg of body weight are life-threatening.
The intoxication symptoms of methanol intoxication run in three phases. Immediately after ingestion of methanol, a narcotic stage appears, as with ethanol , but the intoxicating effect is less than with ethanol. After the latency phase, headache, weakness, nausea, vomiting, dizziness and accelerated breathing occur in connection with the developing metabolic acidosis . The third phase, acidosis, is characterized by damage to the nerves, particularly the optic nerve (nervus opticus) . Visual disturbances that can recede are initially caused by edema on the retina . The degeneration of the optic nerve - in this case toxic optic neuropathy - subsequently leads to blindness . This damage is irreversible. Death can occur as a result of respiratory paralysis .
To treat methanol poisoning, the breakdown of methanol in the human body is prevented so that the toxic by-products do not arise. For this purpose, about 0.7 g of ethanol (commonly: alcohol) per kg of body weight can be administered, which competitively inhibits the degradation of methanol , since the enzyme has a higher affinity for ethanol and thus degrades it preferentially ( substrate specificity ). For effective therapy, the ethanol level has to be maintained for days, depending on the degree of poisoning and the physical condition of the poisoned person. It is more effective to take the ADH inhibitor 4-methylpyrazole ( fomepizole ), which also competitively inhibits the breakdown of methanol. At the same time, the breakdown of formic acid in the body can be promoted by administering folic acid . With sodium bicarbonate the acidification of the body (can acidosis ) are opposed. In the case of severe poisoning or special illnesses such as cirrhosis of the liver or similar, hemodialysis may be necessary. Treatment must be continued until the level of methanol in the blood has dropped below a certain limit.
By the Regulation no. 110/2008 of the European Parliament and of the Council of the methanol content of various alcoholic beverages in which the European Union is limited. In the case of a fruit pomace brandy, for example, a methanol content of 15 g · l −1 (calculated on the pure ethanol content) is the upper limit.
In rare cases alcoholic beverages can contain increased amounts of methanol as a result of improper mashing , fermentation and distillation or freezing out . Most of the known cases of methanol poisoning, for example during the prohibition or the methanol wine scandal in 1986, can be traced back to the consumption of drinking alcohol that was consciously or unconsciously mixed with methanol.
In 2012, the EU included methanol in the Community's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of methanol were concerns about consumer use , environmental exposure, exposure of workers and widespread use as well as the hazards arising from a possible assignment to the group of CMR substances. The re-evaluation took place from 2012 and was carried out by Poland . A final report was then published.
proof

Methanol has an alcohol-like odor. If methanol is mixed with borax and ignited, the resulting trimethyl borate burns with an intense green flame. This reaction works with similar results, but less intense green color, also with ethanol with the addition of concentrated sulfuric acid . That is why ethanol and methanol can be differentiated with this so-called borax sample.
Methanol is often detected using gas chromatographic methods, such as flame ionization detection , or coupled mass spectrometry . Depending on the origin of the sample, it is either concentrated or extracted beforehand using various methods. To detect methanol in the air, it is first passed over silica gel or activated carbon in order to adsorb and concentrate the methanol. The methanol is released again through subsequent thermal desorption . In the case of liquid samples, e.g. for detection in fuel, the sample is first extracted with ethylene glycol , for example , and then analyzed by gas chromatography. Extraction with water is possible for solid samples.
Production systems can be monitored directly during the manufacturing process using infrared spectroscopy . Another method is the oxidation of methanol with strong oxidizing agents, such as potassium permanganate , to formaldehyde, which can be detected using conventional methods.
literature
- Holger Menrad, Alex König: Alcohol Fuels . Springer, Vienna / New York 1982, ISBN 3-211-81696-8 .
- The Federal Minister for Research and Technology (ed.): Development lines in automotive engineering and road traffic . Research reports 1977 to 1985, TÜV Rheinland, Cologne.
- F. Asinger : Methanol, chemical and energy raw material . Akademie-Verlag, Berlin, 1987, ISBN 3-05-500341-1 .
- GA Olah, A. Goeppert, GK Surya Prakash: Beyond oil and gas: the methanol economy. Verlag Wiley-VCH ( limited preview in Google book search)
- Bernd Höhlein: New energy carriers for traffic: methanol and alcohol mixtures , Verlag Forschungszentrum Jülich, 1991, ISBN 3-89336-068-9 .
- VDI book: Energy handbook: Generation, conversion and use of energy. Springer Verlag, 2002, ISBN 3-540-41259-X .
- Klaus Weissermel , Hans-Jürgen Arpe : Industrial Organic Chemistry: Important Raw Materials and Intermediates. Wiley-VCH Verlag 2003, ISBN 3-527-30578-5 , p. 30 ff.
- Martin Bertau, Heribert Offermanns, Ludolf Plass, Friedrich Schmidt, Hans-Jürgen Wernicke: Methanol: The Basic Chemical and Energy Feedstock of the Future: Asinger's Vision Today , 750 pages, Verlag Springer; 2014, ISBN 978-3-642-39708-0 .
- Hochhaus, Karl-Heinz: Alternative fuels in maritime shipping; in Schiffs-Ingenieur Journal No. 2/2017.
Web links
- The octane-increasing effect of methanol in gasoline (source Menrad, motorlexikon.de)
- Methanol Institute, Arlington (English).
- Entry to methanol . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD .
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r s Entry on methanol in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 459.
- ↑ Entry on methanol. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-326.
- ↑ Entry on methanol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 67-56-1 or methanol ), accessed on September 13, 2019.
- ↑ CRC Handbook, pp. 5–20 ( Memento from April 26, 2015 in the Internet Archive ).
- ^ Methanol on the Gelsenchem website accessed on February 28, 2020.
- ↑ Power to Methanol - as long-term storage indispensable for climate protection - Solarenergie-Förderverein Deutschland (SFV) - solar energy, photovoltaics, solar thermal energy, wind energy, geothermal energy, hydropower, biomass residues and electricity storage for the energy transition. Retrieved September 8, 2019 .
- ↑ A. Gossauer: Structure and reactivity of biomolecules. Verlag Wiley-VCH, p. 176 ( limited preview in Google book search).
- ↑ a b G. A. Olah, A. Goeppert, GK Surya Prakash: Beyond oil and gas: the methanol economy. Verlag Wiley-VCH, 2009, ISBN 978-3-527-32422-4 .
- ↑ S. Lee: Methanol synthesis technology. CRC Press, 1990, ISBN 0-8493-4610-X .
- ^ JR Couper, OT Beasley, WR Penney: The chemical process industries infrastructure: function and economics. Verlag Marcel Dekker, 2000, ISBN 0-8247-0435-5 .
- ↑ About BASF. History 1902–1924. (No longer available online.) BASF, archived from the original on July 20, 2012 ; accessed on September 6, 2018 .
- ↑ a b B. Höhlein, Th. Grube, P. Biedermann, H. Bielawa, G. Erdmann, L. Schlecht, G. Isenberg, R. Edinger: Methanol als Energieträger ( Memento from August 13, 2016 in the Internet Archive ) ( PDF file; 5.5 MB). In: Writings from Forschungszentrum Jülich. Energy technology series. Volume 28, ISBN 3-89336-338-6 .
- ↑ T. Holst, A. Arneth, S. Hayward, A. Ekberg, M. Mastepanov, M. Jackowicz-Korczynski, T. Friborg, PM Crill, K. Bäckstrand: BVOC ecosystem flux measurements at a high latitude wetland site in Atmos . Chem. Phys., 10, pp. 1617-1634, 2010.
- ^ DJ Jacob, BD Field, Q. Li, DR Blake, J. de Gouw, Carsten Warneke, A. Hansel, A. Wisthaler, HB Singh, A. Guenther: Global budget of methanol: Constraints from atmospheric observations. In: Journal of Geophysical Research . Vol. 110, 2005; doi :: 10.1029 / 2004JD005172 .
- ↑ CC von Dahl, M. Hävecker, R. Schlögl and IT Baldwin: Caterpillar-elicited methanol emission: a new signal in plant – herbivore interactions? in: The Plant Journal , (2006), 46, pp. 948-960; doi: 10.1111 / j.1365-313X.2006.02760.x .
- ↑ a b c E. Kolb: Spirits technology . Behr's Verlag, 2002, ISBN 3-86022-997-4 .
- ↑ Methanol: How safe are ... the inspection offices for food control and animal health Baden-Württemberg, accessed on September 6, 2018 .
- ↑ HG Classen, PS Elias, M. Winter: Toxicological-hygienic assessment of food ingredients and additives. Behr's Verlag, 2001, ISBN 3-86022-806-4 .
- ^ W. Helferich, CK Winter: Food Toxikology. CRC Press, 2000, ISBN 0-8493-2760-1 .
- ↑ L. Harvey-Smith, RJ Cohen: Discovery of large-scale masers in W3 (OH), Triggered Star Formation in a Turbulent ISM, Proceedings IAU Symposium No. 237, 2006, doi: 10.1017 / S1743921307002104 .
- ↑ ES Wirström1, CM Persson1, A. Hjalmarson1, JH Black, P. Bergman1, WD Geppert, M. Hamberg, E. Vigren: Observational constraints on the formation of interstellar methanol. Organic Matter in Space, Proceedings IAU Symposium No. 251, 2008, doi: 10.1017 / S1743921308021406 .
- ↑ Spitzer Spectra of Protoplanetary Disks at caltech.edu
- ^ Excerpt from the Methanol & Derivatives Global Outlook 2000–2012. ( Memento from July 24, 2012 in the web archive archive.today )
- ↑ China's petrochemicals are booming. (PDF; 99 kB) Dechema eV, February 2007, accessed on September 6, 2018 .
- ↑ a b c d e f g h i F. Asinger: Methanol, chemical and energy raw material . Akademie-Verlag, Berlin, 1987, ISBN 3-05-500341-1 .
- ↑ The 5% solution ( Memento from November 29, 2014 in the Internet Archive ) , at methanol.org, August 2009.
- ^ M. Hennecke: The engineering knowledge. Verlag Springer, Berlin 2007, ISBN 978-3-540-71851-2 .
- ↑ H. Daniel: Physics: Mechanics, waves, heat . Verlag De Gruyter, 1997, ISBN 3-11-015602-4 ( limited preview in Google book search).
- ↑ a b c d e f g h i Technical information and safety data sheet for handling methanol ( Memento of August 26, 2011 in the Internet Archive ) (PDF file; 578 kB)
- ↑ A. Töpel: Chemistry and Physics of Milk: Natural Material - Raw Material - Food . Behr's Verlag, 2004, ISBN 3-89947-131-8 , p. 65 ( limited preview in the Google book search).
- ↑ L. Bergmann, T. Dorfmüller, C. Schaefer: Textbook of Experimental Physics: Mechanics, Relativity, Warmth . Verlag de Gruyter, 1998, ISBN 3-11-012870-5 ( limited preview in the Google book search).
- ^ S. Lee, JG Speight, SK Loyalka: Handbook of alternative fuel technologies. Publisher CRC Press, 2007, ISBN 978-0-8247-4069-6 ( limited preview in Google Book Search).
- ↑ NMR-002: Sample Devices and Magnetic Susceptibility
- ↑ Lange's Handbook of Chemistry. 10th edition, pp. 1669-1674.
- ↑ a b c d Entry on Methyl alcohol (Condensed phase thermochemistry data). In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed November 17, 2019.
- ↑ Lange's Handbook of Chemistry. 10th edition. Pp. 1522-1524.
- ↑ Competition Science Vision , Vol. 3, No. 25, March 2000 ( limited preview in the Google book search).
- ↑ Ambrose, D .; Sprake, CHS: Thermodynamic Properties of Organic Oxygen Compounds. XXV. Vapor Pressures and Normal Boiling Temperatures of Aliphatic Alcohols , in: J. Chem. Thermodyn. 2, pp. 631-645 (1970).
- ^ A b L. Pauling: The nature of the chemical bond , 3rd edition, Verlag Chemie, Weinheim 1973, p. 443.
- ↑ Technical Information & Safe Handling Guide for Methanol ( Memento from September 16, 2012 in the Internet Archive ) (PDF file; 1.6 MB)
- ↑ KJ Tauer, WN Lipscomb: On the crystal structures, residual entropy and dielectric anomaly of methanol. In: Acta Crystallographica . 1952, 5, pp. 606-612, doi: 10.1107 / S0365110X52001696 .
- ↑ alcohols. (PDF; 303 kB) www.uni-tuebingen.de, archived from the original on June 26, 2013 ; Retrieved January 10, 2010 .
- ↑ EV Ivash, DM Dennison, Journal of Chemical Physics 1953, 21, pp 1804th
- ↑ S. Hauptmann: Reaction and Mechanism in Organic Chemistry. Verlag Teubner, p. 61 ( limited preview in Google book search).
- ^ Klaus Weissermel, Hans-Jürgen Arpe: Industrial Organic Chemistry: Important Raw Materials and Intermediates. Wiley-VCH Verlag, 2003, ISBN 3-527-30578-5 .
- ↑ GW Becker, D. Braun, L. Bottenbruch: Kunststoffhandbuch. 11 volumes in 17 parts, volume 3/1, technical thermoplastics. BD 3 / Part 1, Verlag Hanser Fachbuch, 1992, ISBN 3-446-16368-9 .
- ↑ J. Buddrus: Fundamentals of organic chemistry. Gruyter publishing house, 2011, ISBN 978-3-11-024894-4 .
- ↑ a b c d e http://www.methanol.org/Methanol-Basics/Resources/MMSA-Global-Methanol-Supply-and-Demand.aspx (link not available)
- ↑ Rectisol Process ( Memento from June 16, 2012 in the Internet Archive )
- ^ ICIS : Formaldehyde Uses and Market Data
- ^ Formaldehyde ( Memento from August 27, 2013 in the Internet Archive ), from MMSA.
- ^ Formaldehyde Uses and Market Data. ICIS , accessed January 9, 2010 .
- ↑ A. Behr : Aliphatic Intermediates. In: Roland Dittmeyer, Wilhelm Keim , Gerhard Kreysa , Alfred Oberholz (eds.): Winnacker, Küchler. Chemical Technology: Processes and Products Volume 5: Organic Intermediate Compounds, Polymers. WILEY-VCH Verlag, Weinheim, ISBN 3-527-30770-2 .
- ↑ a b W.Keim , A. Behr, G. Schmitt: Fundamentals of Industrial Chemistry: techn. Products and Processes. 1st edition Salle, Frankfurt / Berlin / Munich 1986, ISBN 3-7935-5490-2 (Sauerländer, ISBN 3-7941-2553-3 ).
- ↑ An Investigation Of The Feasibility Of Coal-Based Methanol For Application In Transportation Fuel Cell Systems ( Memento of February 3, 2013 in the Internet Archive ) (PDF file; 766 kB)
- ↑ Power to Methanol - as long-term storage indispensable for climate protection - Solarenergie-Förderverein Deutschland (SFV) - solar energy, photovoltaics, solar thermal energy, wind energy, geothermal energy, hydropower, biomass residues and electricity storage for the energy transition. Retrieved September 8, 2019 .
- ^ Athanasios A. Tountas, Xinyue Peng, Alexandra V. Tavasoli, Paul N. Duchesne, Thomas L. Dingle: Towards Solar Methanol: Past, Present, and Future . In: Advanced Science . tape 6 , no. 8 , 2019, ISSN 2198-3844 , p. 1801903 , doi : 10.1002 / advs.201801903 , PMID 31016111 , PMC 6468977 (free full text).
- ↑ Overview of the use of methanol in rocket and aircraft fuels
- ↑ Tom B. Reed, RM Lerner: Methanol: A Versatile Fuel for Immediate Use. In: Science , 182.4119, 1973, pp. 1299-1304; doi: 10.1126 / science.182.4119.1299 .
- ↑ alcohol fuel , umweltlexikon-online.de
- ↑ POWERED BY METHANOL
- ↑ H. Heitland: Alternatives in traffic: assessment of their chances and risks by PC simulation models. Verlag Frank Timme, p. 65 ( limited preview in Google book search).
- ↑ Inst. Francais Du Petrole (Ed.): VII. International Symposium on Alcohol Fuels. Editions Technip, ISBN 2-7108-0517-0 , p. 277 ( limited preview in Google book search).
- ↑ S. Geitmann: Renewable Energies and Alternative Fuels. With new energy into the future. Verlag Hydrogeit, 2005, ISBN 3-937863-05-2 .
- ↑ JJ Romm: The hydrogen boom: desire and reality in the race for climate protection. Wiley-VCH Verlag, 2006, ISBN 3-527-31570-5 .
- ↑ B. Aldrich: ABC's of Afv's: A Guide to Alternative Fuel Vehicles. Diane Pub, 1995 ISBN 0-7881-4593-2 ( limited preview in Google Book Search).
- ↑ M. Trzesniowski: racing car technology: basics, construction, components, systems. Vieweg + Teubner Verlag, 2008, ISBN 978-3-8348-0484-6 .
- ↑ Ulrike Schramm: SFC Energy and Beijing Green Century Technologies sign partnership agreement for EFOY Pro fuel cells in China. In: Investors, press release. SFC Energy AG, April 24, 2018, accessed on May 18, 2018 .
- ↑ MTBE Fact Sheet # 3 Use And Distribution Of MTBE And Ethanol (PDF file; 20 kB)
- ↑ Biodiesel production capacities in Germany
- ↑ K. Liu, C. Song, V. Subramani: Hydrogen and Syngas Production and Purification Technologies: Hydrocarbon Processing for H 2 Production. Verlag John Wiley & Sons, 2010, ISBN 978-0-471-71975-5 , p. 510 ff.
- ↑ As long as R. Błaszkowski, Rutger A. van Santen: Theoretical Study of CC Bond Formation in the methanol-to-gasoline process. (PDF file; 222 kB) In: J. Am. Chem. Soc. 1997, 119, pp. 5020-5027; doi: 10.1021 / ja963530x .
- ↑ Michael Seiler, Udo Schenk, Michael Hunger: Conversion of methanol to hydrocarbons on zeolite HZSM-5 investigated by in situ MAS NMR spectroscopy under flow conditions and on-line gas chromatography. In: Catalysis Letters . 1999, 62, pp. 139-145; doi: 10.1023 / A: 1019086603511 .
- ↑ ThyssenKrupp Base: Methanol ( Memento from July 8, 2012 in the web archive archive.today )
- ↑ MTBE in Fuels , from EPA.gov.
- ↑ MTBE Ban in California (PDF file; 674 kB), from GAO.gov.
- ↑ Environmental relevance of the substance methyl tertiary butyl ether (MTBE) with special consideration of water protection , from Umweltbundesamt.de.
- ↑ a b Annual Technical Progress Report for Project Entitled “Impact of DME-Diesel Fuel Blend Properties on Diesel Fuel Injection Systems” May 16, 2003 .
- ↑ Patent US4341069 : Method for generating power upon demand.
- ↑ Environment, energy and transport (PDF file; 1.1 MB) EU research results in the field of urban and regional transport.
- ^ DME, Clean Fuel for Transportation , at International DME Association.
- ↑ BioDME. BioDME, accessed September 6, 2018 .
- ↑ Patent US5264553 : Method of forming uniform polymer spheres, composite particles and polymer encapsulated particles.
- ↑ BEA Jacobs: Design of slurry transport systems. Verlag Elsevier, p. 254 ( limited preview in Google book search).
- ↑ Process for the separation of aromatic hydrocarbons from mixtures ( Memento of January 28, 2012 in the Internet Archive ) (PDF file; 424 kB).
- ↑ http://www.eawag.ch/about/haben/homepages/buergmhe/Stoffwechsel08/Stoffwechsel_Termin_6-2009.pdf (link not available)
- ↑ H. Kloosterman, JW Vrijbloed, and L. Dijkhuizen (2002): Molecular, biochemical, and functional characterization of a Nudix hydrolase protein that stimulates the activity of a nicotinoprotein alcohol dehydrogenase . In: J Biol Chem . 277 (38), pp. 34785-34792; PMID 12089158 ; PDF (free full text access)
- ↑ Georg Fuchs (Ed.), Hans. G. Schlegel (Author): General Microbiology. 8th edition. Thieme Verlag, Stuttgart; 2007, ISBN 3-13-444608-1 , p. 311.
- ↑ Cleanthis J. Israelidis: Nutrition - Single Cell Protein, Twenty Years Later. www.biopolitics.gr, archived from the original on October 7, 2011 ; accessed on September 6, 2018 .
- ↑ External identifiers of or database links for methanol-d1 : CAS number: 1455-13-6, EC number: 215-933-0, ECHA InfoCard: 100.014.485 , PubChem : 123113 , ChemSpider : 109729 , Wikidata : Q82908334 .
- ↑ External identifiers of or database links for Methanol-d3 : CAS number: 1849-29-2, EC number: 217-435-9, ECHA InfoCard: 100.015.851 , PubChem : 123132 , ChemSpider : 109747 , Wikidata : Q83041789 .
- ↑ External identifiers or database links for methanol-d4 : CAS number: 811-98-3, EC number: 212-378-6, ECHA InfoCard: 100.011.253 , PubChem : 71568 , ChemSpider : 64640 , Wikidata : Q1100804 .
- ↑ R. Kavet, KM Nauss: The Toxicity of Inhaled Vapors methanol. (PDF file; 2.2 MB) In: Critical Reviews in Toxicology . 1990.
- ^ GF Fuhrmann: Toxicology for natural scientists. Vieweg + Teubner Verlag, 2006, ISBN 3-8351-0024-6 , p. 269.
- ^ B. Madea, B. Brinkmann: Handbook of judicial medicine, Volume 2. Verlag Springer, ISBN 3-540-66447-5 , p. 523 ( limited preview in Google book search).
- ^ PU Fechner, KD Teichmann: Medicinal eye therapy: Basics and practice. Georg Thieme Verlag, 2000, ISBN 3-13-117924-4 , pp. 516-517.
- ^ Methanol poisoning - Effective therapy with formepizole - GFI
- ↑ Regulation (EC) No. 110/2008 of the European Parliament and of the Council of January 15, 2008 on the definition, description, presentation and labeling of spirits as well as the protection of geographical indications for spirits and the repeal of Regulation (EEC) No. 1576 / 89
- ↑ Stuart A. Schneck: Methyl, alcohol. (PDF file; 763 kB) In: Handbook of Clinical Neurophysiology. Vol. 36, 1979, pp. 351-360.
- ↑ Wine: Stunning idea . In: Der Spiegel . No. 16 , 1986, pp. 130-132 ( Online - Apr. 14, 1986 ).
- ^ European Chemicals Agency (ECHA): Substance Evaluation Report and Conclusion Document .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Methanol , accessed on March 26, 2019.
- ↑ G. Blumenthal, D. Linke, S. Vieth: Chemistry: Basic knowledge for engineers. Vieweg + Teubner Verlag, 2006, ISBN 3-519-03551-0 , p. 242.
- ^ PJ Baugh: Gas Chromatography: A User-Oriented Representation . Publisher Vieweg ( limited preview in Google Book Search).
- ^ Chemical Properties of Methanol ( Memento of March 10, 2010 in the Internet Archive )