Methacrylic acid methyl ester
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
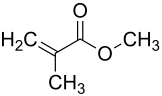
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Methacrylic acid methyl ester | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 8 O 2 | ||||||||||||||||||
| Brief description |
colorless liquid with a characteristic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 100.12 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.94 g cm −3 |
||||||||||||||||||
| Melting point |
−48.2 ° C |
||||||||||||||||||
| boiling point |
101 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
slightly soluble in water (15 g l −1 at 20 ° C) |
||||||||||||||||||
| Refractive index |
1.414 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
DFG / Switzerland: 50 ml m −3 or 210 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Methacrylic acid methyl ester ( methyl methacrylate , MMA ) is a colorless liquid with an unpleasant ester-like odor. MMA is highly flammable, evaporates easily and has a boiling point of 101 ° C. Mixed with water, the boiling point of MMA drops to 83 ° C to form an azeotropic mixture. MMA has the UN number 1247.
Manufacturing
MMA is mainly produced from acetone cyanohydrin , which is reacted with sulfuric acid to form methacrylic acid amide and then esterified . Acetone cyanohydrin is made from acetone and hydrogen cyanide .
Methyl methacrylate can also be produced industrially by the two-stage oxidation of isobutene and without the use of hydrogen cyanide. However, this so-called C 4 process is used less often. Isobutene itself by dehydration of tert -butanol or by cleavage of methyl tert-butyl ether ( MTBE produced), both processes run in the gas phase, are typically catalyzed by heterogeneous contacts and give a good yield of isobutene. The first stage of the oxidation leads to methacrolein ; the catalysts used are similar or are derived from the contacts that are also used for propene oxidation to acrolein.
Methacrylic acid methyl ester is stabilized after production to avoid spontaneous polymerization.
Alpha process
A more recent method, which was implemented in 2008, is based on ethylene as a raw material of, the homogeneously catalyzed with carbon monoxide and methanol in one step is carboxymethylated, there arises extremely efficiently with a high turnover number (TOF) and turnover number (TON, a measure of the Efficiency of a catalyst before it is consumed by side reactions or other effects) methyl propionate as an intermediate product. In the next step, methyl propionate is aldolized and dehydrated in the gas phase at a special contact with formaldehyde , which directly results in MMA. The process was originally developed by ICI , then brought to technical maturity by Lucite International and finally a production facility with an annual capacity of 120,000 tons was built by MRC-Lucite in Singapore. The process is referred to in the literature as the alpha process or alpha process.
Hydroesterification of ethylene with carbon monoxide and methanol to methyl propionate (methyl propionate):
Condensation of methyl propionate and formaldehyde to methyl methacrylate and water:
A process that has not yet been implemented by Lucite (now MRC-Lucite) as the β process is based on propyne as the starting material. A catalytic conversion of propyne with methanol and carbon monoxide, similar to the alpha process, is used to convert directly into MMA in a single stage. Despite its high catalytic efficiency, the process has not yet been used, as propyne is not a common, traded petrochemical raw material and is hardly available.
LiMA process
The latest development step is the LiMA (“Leading in Methacrylates”) process, with which Evonik Industries went public in 2017.
Like the alpha process, the LiMA process is also a C 2 process based on the raw material ethylene , with the ethylene being hydroformylated to propionaldehyde in an oxo synthesis with synthesis gas at a rhodium contact .
In the first step ( A ) of the process propionaldehyde with LiMA is formalin HCHO in a Mannich reaction with dimethylamine / acetic acid -Katalysatorgemisch to methacrolein reacted.
The conversion of the starting materials is practically quantitative, the selectivity to methacrolein is over 98%.
In the second step ( B ), methacrolein reacts with atmospheric oxygen and methanol on a nickel - gold contact at low temperature (approx. 90 ° C) and pressure (approx. 6 bar) to form methyl methacrylate with methacrolein conversions of approx. 70% and MMA selectivities > 95%.
The process has so far only been tested on a pilot scale, but should be more cost-effective and more environmentally friendly than the Alpha process.
properties
Physical Properties
Methacrylic acid methyl ester is a colorless liquid with a melting point of −48.2 ° C and a boiling point of 101 ° C at normal pressure . According to Antoine, the vapor pressure function results according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 5.37785, B = 1945.56 and C = −7.569 in the temperature range from 312.4 to 362.3 K. Important thermodynamic parameters are given in the following table:
| property | Type | Value [unit] | Remarks |
|---|---|---|---|
| Standard enthalpy of formation | Δ f H 0 liquid Δ f H 0 gas |
−388.8 kJ mol −1 −348.7 kJ mol −1 |
as a liquid as a gas |
| Enthalpy of combustion | Δ c H 0 liquid | −2724.6 kJ mol −1 | as a liquid |
| Heat capacity | c p | 215.3 J mol −1 K −1 (25 ° C) 2.15 J g −1 K −1 (25 ° C) |
as a liquid |
| Enthalpy of fusion | Δ f H | 13.451 kJ mol −1 | at the melting point |
| Enthalpy of evaporation | Δ V H | 33.3 kJ mol −1 | at normal pressure boiling point |
Chemical properties
Methacrylic acid methyl ester can polymerize spontaneously , especially if it contains impurities . Due to the Trommsdorff effect, there is a sudden increase in temperature, which is accompanied by an increase in pressure. This polymerization can be started in a targeted manner by adding initiators , usually peroxides . The heat of polymerization is −59 kJ · mol −1 or -590 kJ · kg −1 .
Safety-related parameters
Methacrylic acid methyl ester forms highly flammable vapor-air mixtures. The compound has a flash point of 10 ° C. The explosion range is between 1.7% by volume (70 g / m 3 ) as the lower explosion limit (LEL) and 12.5% by volume (520 g / m 3 ) as the upper explosion limit (UEL). The maximum explosion pressure is 8.6 bar. The limit gap width was determined to be 0.95 mm. This results in an assignment to explosion group IIA. The ignition temperature is 430 ° C. The substance therefore falls into temperature class T2.
use
Methacrylic acid methyl ester is mainly used for the production of acrylic glass . Furthermore, MMA is, as a rule, the main component of every plastic dental prosthesis . Liquid MMA is mixed with granulated PMMA (polymethyl methacrylate) to form a viscous dough and hardened (polymerized). However, it is also used in the production of bone cement for cementing artificial joints, in paint production and as a two-component adhesive ( methyl methacrylate adhesive ).
Risk assessment
In 2013, methacrylic acid methyl ester was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The causes of the uptake of methyl methacrylate were concerns about consumer use , exposure of sensitive population groups , high (aggregated) tonnage, high risk characterization ratio (RCR) and widespread use as well as the suspected hazards from sensitizing properties. The re-evaluation took place from 2014 and was carried out by France . A final report was then published.
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r s t u Entry for CAS no. 80-62-6 in the GESTIS substance database of the IFA , accessed on March 14, 2017(JavaScript required) .
- ↑ Data sheet methyl methacrylate from Sigma-Aldrich , accessed on April 10, 2011 ( PDF ).
- ↑ Entry on methyl methacrylate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 80-62-6 or methyl methacrylate ), accessed on November 2, 2015.
- ↑ Entry on methacrylic acid ester. In: Römpp Online . Georg Thieme Verlag, accessed on September 23, 2014.
- ^ Mark W. Hooper: Considerations of Industrial Fine Chemical Synthesis. In: SM Roberts, J. Xiao, J. Whittall, T. Pickett (Eds.): Catalysts for Fine Chemical Synthesis. Vol. 3: Metal Catalysed Carbon-Carbon Bond-Forming Reactions. John Wiley & Sons, 2004, ISBN 0-470-86199-1 . (PDF)
- ↑ Best in class: Evonik develops the most efficient process for the production of methyl methacrylate. In: Press release Evonik. Evonik, October 5, 2017, accessed June 10, 2020 .
- ^ How we develop the best process for methyl methacrylate. In: Presentation by Steffen Krill. Evonik, October 5, 2017, accessed June 10, 2020 .
- ↑ Patent WO2014170223A1 : Process for the production of methyl methacrylate. Registered on April 11, 2014 , published on October 23, 2014 , applicant: Evonik Industries AG, inventors: S. Krill, T. Balduf, M. Köstner, M. Grömping, A. Lygin, R. Burghardt.
- ^ Methyl Methacrylate (MMA) Production by Evonik LiMA ™ Process. In: PEP Review. IHS Markit, May 2018, accessed June 10, 2020 .
- ↑ By A. Brockhaus, Jenckel, E .: About the kinetics of the thermal degradation of polymethacrylic acid methyl ester. In: Makromol. Chem. 18, 1956, pp. 262-293, doi: 10.1002 / macp.1956.020180124 .
- ↑ a b c R. Vilcu, S. Perisanu: The ideal gas state enthalpies of formation of some monomers. In: Rev. Roum. Chim. 25, 1980, pp. 619-624.
- ↑ M. Karabaev, TP Abduzhaminov, AA Saidov, Kh. T. Igamberdyev: Thermophysical properties of liquid methacrylic acid and its simple esters. In: Izv. Akad. Nauk Uzb. SSR Ser. Fiz.-Mat. Nauk. 4, 1985, pp. 71-74.
- ↑ M. Karabaev, AA Saidov, TP Abduzhaminov, MM Kenisarin: Heat capacity and molecular kinetic processes of condensed phase acrylates and methacrylates. In: Izv. Akad. Nauk UzSSR, Ser. Fiz.-Math. 6, 1985, pp. 51-54.
- ↑ MK Karabaev, TP Abduzhaminov, MM Kenisarin, AA Saidov: Thermodynamics of the crystal-liquid phase transition in acrylates and methacrylates. In: Izv. Akad. Nauk Uzb. SSR, Ser. Fiz.-Mat. Nauk. 5, 1985, pp. 74-77.
- ↑ WV Steele, RD Chirico, AB Cowell, SE Knipmeyer, A. Nguyen: Thermodynamic Properties and Ideal-Gas Enthalpies of Formation for trans -Methyl Cinnamate, α-Methyl Cinnamaldehyde, Methyl Methacrylate, 1-Nonyne, Trimethylacetic Acid, Trimethylacetic Anhydride, and Ethyl Trimethyl Acetate. In: J. Chem. Eng. Data . 47, 2002, pp. 700-714, doi: 10.1021 / je010086r .
- ↑ Employer's liability insurance association for raw materials and chemical industry , leaflet R 008 Polyreactions and polymerizable systems. Edition 05/2015, ISBN 978-3-86825-069-5 , p. 26.
- ↑ a b c d E. Brandes, W. Möller: Safety parameters: Volume 1: Flammable liquids and gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Methyl methacrylate , accessed on March 26, 2019.





