Ethene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethene ( IUPAC ) | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 4 | |||||||||||||||
| Brief description |
extremely flammable, colorless gas with a slightly sweet odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 28.05 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
1.178 kg m −3 (15 ° C) |
|||||||||||||||
| Melting point |
−169.18 ° C |
|||||||||||||||
| boiling point |
−103.8 ° C |
|||||||||||||||
| Vapor pressure |
4.1 M Pa (20 ° C) |
|||||||||||||||
| solubility |
very bad in water (130 mg l −1 ) |
|||||||||||||||
| Dipole moment |
0 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 10,000 ml m −3 or 11,500 mg m −3 |
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
52.4 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ethene (also ethene , ethylene or ethylene ) is a gaseous, colorless, flammable, sweet-smelling organic compound with the empirical formula C 2 H 4 . It is the simplest alkene , an unsaturated hydrocarbon having a carbon -Kohlenstoff- double bond .
Ethene is produced commercially by the steam cracking of a variety of hydrocarbons . In Europe and Asia , ethene is mainly produced from naphtha or gas oil , in the United States , Canada and the Middle East also from ethane , propane and liquefied gas .
Ethene is the most widely produced organic basic chemical and is used for the manufacture of primary secondary products such as polyethylene , ethylene oxide , styrene and α-olefins .
Plants such as ripe apples and bananas and the Japanese red pine emit ethene, and it is important as a ripening gas and phytohormone .
history
In ancient Egypt, ethene was unconsciously used to ripen mulberry figs . For this purpose, the unripe fruits were scratched. These then produced ethene and accelerated the ripening process. Ethene was first mentioned as a gas in 1669 in the work Actorum Laboratorii Chymici Monacensis, seu Physicae subterraneae by the German alchemist Johann Joachim Becher . Beaker obtained the gas by heating ethanol with sulfuric acid .
As early as 1777, the Dutch physician Jan Ingenhousz is said to have learned about the synthesis of ethene carried out in Amsterdam by Henricus Aeneae (Enée) and his colleague John Cuthbertson. In 1795, the four Dutch chemists Johan Rudolph Deiman , Adriaan Paets van Troostwijk , Anthonie Lauwerenburgh and Nicolaas Bondt discovered the synthesis of 1,2-dichloroethane from ethene and chlorine . Since the product was called the oil of the Dutch chemists , ethene was called in French gaz oléfiant ("oil-forming gas"), in English olefiant gas . In 1807, John Dalton tried to create a structural formula. In the second half of the 19th century, the synthetic preparation of vegetable acids such as succinic acid from ethene succeeded.
Technically ethene was first by dehydration of ethanol or by isolation from coke oven gas recovered. The first large-scale production of ethene from ethanol took place in 1913 in the electrochemical works in Bitterfeld. The ethene obtained in this way was used for cooling purposes. It was not until 1981 that the process was resumed in Brazil by Salgema Industrias Quimicas. Salgema produced around 100,000 tons per year based on a Petrobras patent . The starting material for the "Green Ethylene" is ethanol from sugar cane . In 2010, Braskem built a plant for the dehydration of ethanol in Triunfo in Rio Grande do Sul with an annual capacity of 200,000 tons.
The technically relevant process today is the steam cracking of naphtha or higher hydrocarbon mixtures such as hydrowax . Germany is in Europe with a production of 2.9 million tonnes (1989) is the largest Ethenhersteller, followed by France with 2.5 and England with 1.9 million tons.
Ethene can be obtained under suitable process conditions using alumina-titanium dioxide catalysts with dehydration from methanol or its secondary product dimethyl ether . In the laboratory it is dehalogenation of 1,2-dichloroethane with zinc gained.
Extraction and presentation
Ethene is partially obtained as a by- product , but the majority of the ethene required is obtained by thermal or catalytic cracking of hydrocarbons. Natural gas , naphtha or higher-boiling distillate cuts are usually used as raw materials . In addition to cracking, the dehydration of ethane , which is found in large quantities in shale gases, is a technical route. The dehydration of ethanol plays an important role in countries with large bioethanol production .
In 2010 around 123 million tonnes of ethene were produced worldwide . Production in Germany was 5.1 million tons per year.
Ethene distribution
In Germany and parts of the Netherlands there is an ethene pipeline system for transport between the chemical sites from Rotterdam via Antwerp to the Cologne area and the Emscher-Lippe area as well as the Rhine-Main area and Ludwigshafen am Rhein.
In Bavaria there is a pipeline between the chemical triangle in the south-east and the chemical site near Ingolstadt ( ethylene pipeline Münchsmünster-Gendorf ). With the commissioning of the 370 km long ethylene pipeline south from Münchsmünster to Ludwigshafen am Rhein in July 2013, the north-west German network was connected with the Bavarian chemical sites.
The industrial sites of Brunsbüttel and Stade , north and south of the Elbe , are to be connected with a 54-kilometer chemical and gas pipeline. In Stade there is a connection to an ethene pipeline to Böhlen in Saxony.
properties
Molecular properties
Between the two carbon - atoms is a double bond . A rotation around this bond requires considerably more energy than a rotation around a single bond between two carbon atoms. Due to the sp 2 - hybridization of the carbon atoms is the molecule planar , i.e., all the atoms lie in a plane. The H − C − H bond angles are each 117 ° and thus deviate only slightly from the theoretically ideal value of the trigonal planar form of 120 °. The C = C double bond, with a bond length of 133 pm, is significantly shorter than the C − C single bond in ethane (154 pm). However, the two bonds between the carbon atoms in ethene are not equally strong: The bond energy of the σ bond is around 450 kJ / mol, that of the π bond is around 270 kJ / mol (see also σ-π model ). Accordingly, the π bond can be split more easily, for example in a chemical reaction. In general, due to the high electron density between the two carbon atoms , ethene is much more reactive than, for example, the simply bound ethane.
Physical Properties
Due to the reactive C = C double bond, addition to this bond is a typical reaction of ethene. Only 130 mg / l ethene is soluble in water, but ethene is readily soluble in organic (non-polar) solvents. Ethene has a slightly sweet, unpleasant odor. The odor threshold is 260 ml / m 3 . The calorific value of ethene is 59.955 MJ / m 3 or 50.9 MJ / kg.
Other features:
- Heat of fusion : 3.35 kJ / mol
- Heat of vaporization : 13.9 kJ / mol
- C p : 42.9 J / (mol K)
- Δ f H 0 g : 52.47 kJ / mol
- S 0 : 219.32 J / (mol K)
Ethene crystallizes at −175 ° C in a rhombic unit cell with two molecules per cell and the lattice parameters a = 6.46, b = 4.87, c = 4.14. The density is 0.717 g / m 3 .
The flash point is −136 ° C, the ignition point is 425 ° C.
Chemical properties
Reactions with the formation of a carbon-carbon bond
Ethene molecules polymerize , radically under high pressure or with the help of Ziegler catalysts to form polyethylene .
Ethene reacts with other olefins such as propylene to form ethylene-propylene copolymers , with the addition of dienes to form ethylene-propylene-diene rubber .
Ethene reacts with tetrafluoroethylene to form ethylene-tetrafluoroethylene , and with other unsaturated compounds to form copolymers.
With nickel catalysis , ethene can be oligomerized to α-olefins. Internal olefins can be converted into α-olefins with ethene with metathesis . Both reactions are used on an industrial scale in the SHOP process .
The hydroformylation of ethene with carbon monoxide and hydrogen is a technical way of preparing propanal .
The hydrocyanation of ethene with hydrogen cyanide to propionitrile is catalyzed by nickel catalysts . Hydrogenation or hydrolysis produces propylamine or propionic acid .
Ethene reacts in an ene reaction , a pericyclic reaction with a compound containing hydrogen in the allylic position, forming a bond between a carbon atom of the double bond and the enophile, transferring the allylic hydrogen to the enophile.
Reactions with the formation of an oxygen-carbon bond
Ethene burns with sufficient oxygen supply to water and carbon dioxide.
With air or oxygen and silver as the catalyst , epoxidation to ethylene oxide takes place at a temperature of 220 to 280 ° C and under increased pressure . Further reaction with water produces ethylene glycol .
Reaction with sulfuric acid produces a sulfuric acid half- ester which can be converted into ethanol by hydrolysis . This synthesis route to industrial alcohol has been replaced by the phosphoric acid catalyzed reaction with water.
In the Wacker-Hoechst process , ethene is oxidized to acetaldehyde using palladium catalysis . The reaction is represented by the following equations:
Organometallic compounds
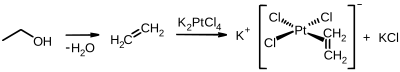
The first organometallic compound with an ethene ligand, the Zeise salt with the empirical formula K [PtCl 3 (C 2 H 4 )] H 2 O, was synthesized in 1830 by the Danish chemist William Christopher Zeise . Ethene forms π complexes with transition metals.
Other reactions
When exposed to heat and in the absence of air, ethene breaks down into methane and carbon.
Ethene reacts with chlorine to form 1,2-dichloroethane ( addition reaction ).
Ethene reacts with hydrogen under high pressure and the presence of metallic catalysts such as platinum and nickel to form ethane ( hydrogenation ).
use

The most important by-products of ethene are polyethylene (56%), i. H. the plastic types HDPE, LDPE and LLDPE, ethylene dichloride for the production of PVC (14%), ethylene oxide (11%) for the production of the polyester precursor ethylene glycol or, for example, nonionic surfactants (detergents) and ethylbenzene (7%) for the production of polystyrene . This means that more than 75% of the ethene is used to make plastics.
Ethene is also the starting material for the production of numerous organic compounds such as anthracene , 2-chloroethanol , chloroethane , propanal , isoprene , vinyl acetate , propanoic acid , butene , styrene , ethanediol and other substances. In the Wacker-Hoechst process , ethene is converted into acetaldehyde (ethanal) on an industrial scale using molecular oxygen (around 1.3% of ethene consumption).
Ethene was used as an anesthetic in addition to nitrous oxide , especially for weak anesthesia. It has a narcotic and muscle relaxing effect. It was used publicly for the first time in Chicago in 1923; the narcotic effect of ethene is somewhat stronger than that of laughing gas and has a similar mechanism of action. However, it is no longer used today because it is flammable and has an unpleasant smell. In addition, the anesthetic effect of ethene is not very good compared to other common narcotics, in order to achieve a good effect the anesthetic mixture would have to contain at least 80% ethene.
It is also used to ripen unripe fruits such as apples , bananas and tomatoes as well as to induce flower formation, either through fumigation in closed greenhouses or outdoors via active ingredients that release ethene in the plant cell, such as. B. ethephon or Etacelasil. Ethene is also a fuel gas and is used for high-speed flame spraying . Ethene in the chemical industry raw material for the synthesis of more than 30% of petrochemicals , it has the ethyne displaced after World War II largely because acetylene is more expensive to manufacture, while ethene obtained masse in industrial processes, is since oil in large quantities available .
It is also used in the manufacture of pesticides and chemical weapons such as mustard gas (2,2-dichlorodiethyl sulfide).
During the Second World War , ethene gas was used experimentally by the Taifun special troop to attack fortresses .
Biological effect
Ethene is a phytohormone (plant hormone). It is synthesized by plants from the amino acid methionine , partially stimulated by the phytohormone auxin . As a hormone, it influences germ growth and senescence in plants . It causes the fruit to ripen , the flowers to develop , the leaves to be shed in autumn and parts of the plant to die off. As a gaseous substance, ethene is mainly found in the spaces between the cells, the intercellular areas .
As early as 1901, Dimitri Neljubow showed that ethene triggers the so-called “triple response” in plants. This occurs with germinating roots that are fumigated with ethene. The effect was noticeable on plants on defective city gas lines, which showed unusual growth. It is about an inhibition of the longitudinal growth in cooperation with a thickening of the stem and a deactivation of the gravitropism , i.e. the growth in the direction of the gravitational force . This effect comes about through a reorientation of the microtubules , which, as skeletal structures, specify the direction of growth (accumulation of cellulose fibers ) of the germ. They are brought from a vertical to a horizontal orientation. The overcoming of obstacles is assumed to be the biological meaning: Ethene is formed during the entire growth process and accumulates in front of obstacles, at which there is a growth in thickness and thus a greater development of force through the root tip.
The second function of ethene relates to various aging processes in the plant. This includes the ripening of fruits and the development of flowers as well as the shedding of leaves ( abscission ) or the death of parts of plants ( senescence ). Important for these functions is the avalanche-like increase in the available amount of ethene, which comes about because the synthesis of ethene is activated by its presence. In this way, a fruit ripens in all places at the same time. The effect of the ripening of fruits is used in agriculture in order to subsequently induce unripe harvested fruits to metabolic processes that allow the fruits to ripen.
Since the mid-1990s, targeted genetic modifications have been used to produce tomatoes that are particularly durable ( Flavr-Savr tomatoes ). The gene responsible for the production of ethene is switched off. These tomatoes can then be fumigated with ethene as required and thereby made ripe. Often fruits are not intentionally brought to ripen, then transported, and only ripened at the destination with the help of ethene. Unripe tomatoes can be made ripe by adding a few ripe tomatoes. These secrete ethene and thus ripen the unripe tomatoes.
Ethene is also essential as an "alarm substance" in the event of pest infestation on the plant and in the event of wounds. Together with other substances such as salicylic acid and jasmonate , ethene delimits the affected area and provides plant poisons . As a gas, ethene acts on neighboring plant parts or plants and sets off the alarm cascade there. Acacias communicate danger through the excretion of ethene to nearby acacias when they are grazed by antelopes or giraffes, as a study by Sylvia Hughes shows.
As with other phytohormones, very little research has been carried out into the mechanism of action of ethene. It is assumed that ethene acts on specific receptor molecules (ethene receptor ETR) on the cell membranes , which initiate a cascade of effects within the cell . Specifically, this is the inactivation of the serine / threonine kinase CTR1. The signal is passed on to different cell nucleus - proteins (IN3 / EIL proteins) as transcription factors in gene expression effect and thus certain genes activate. The first known target gene for these proteins has been described as ethene response factor 1 (ERF1). This factor in turn controls several genes, so that when ethene acts on this system, a whole series of genetic activities is always triggered. During fruit ripening, for example, various enzymes have to be formed to soften the cell wall, while senescence involves the formation of chitin and cellulose-degrading enzymes ( chitinases , cellulases ). The repertoire for plant stress, i.e. the reaction to pests and wounds triggered by ethene, is very extensive . In this situation, among other things, chitinases (as a defense against insects ), glucanases , proteinase inhibitors (inhibitors for protein-degrading enzymes, against fungi) and many other antibodies are produced.
The synthesis of ethene in the plant starts from the amino acid methionine . This produces in a first step by coupling to adenosine the S-adenosylmethionine (SAM). The ACC oxidase releases ethene from the secondary product 1-aminocyclopropanecarboxylic acid (ACC) . The formation of the oxidase is stimulated by ethene itself, which, like a chain reaction in neighboring cells, promotes ethene formation.
hazards
Ethene burns in the air with a slightly sooty, glowing flame. Ethene is extremely flammable. Ethene containers must be kept in a well-ventilated place. It must be kept away from sources of ignition and measures must be taken to prevent electrostatic charging. It forms explosive mixtures in proportions of 3 to 36 percent by volume in air. Ethene has a narcotic effect in high concentrations.
proof
The double bond of ethene can be detected with the help of bromine water , since the reaction of the two substances consumes the bromine with the formation of 1,2-dibromoethane and thereby the brownish bromine water is decolored.
literature
- Klaus Lürssen: The plant hormone ethylene. Biosynthesis, effects on plants and possible uses. In: Chemistry in Our Time . 15th year, No. 4, 1981, pp. 122-129, doi: 10.1002 / ciuz.19810150405 .
- Claus-Jürgen Estler: Pharmacology and Toxicology . Schattauer, Stuttgart 1992, 2000, ISBN 3-7945-1898-5 .
- Gerhard Luft: High pressure polymerization of ethylene. In: Chemistry in Our Time . 34. Jg., No. 3, 2000, pp. 190-199, doi : 10.1002 / 1521-3781 (200006) 34: 3 <190 :: AID-CIUZ190> 3.0.CO; 2-V .
Web links
- Entry on ethene . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD
Individual evidence
- ↑ a b c d e f g h Entry on ethene in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Permittivity (Dielectric Constant) of Gases, pp. 6-188.
- ↑ Entry on ethylene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 74-85-1 or ethene ), accessed on November 2, 2015.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-22.
- ↑ The Sycomore Fig (Html version) ( Memento from January 8, 2012 in the Internet Archive ) , PDF version (593 kB) from May 30, 2009.
- ^ Winfried R. Pötsch, Annelore Fischer and Wolfgang Müller with the collaboration of Heinz Cassebaum : Lexicon of important chemists . Bibliographisches Institut, Leipzig 1988, ISBN 3-323-00185-0 , pp. 33-34.
- ↑ Appendix, §VIII, p. 474 ff., Experiments and observations relating to the various branches of natural philosophy: with a continuation of the observations on air , Joseph Priestley, London: printed for J. Johnson, 1779, volume 1.
- ^ Seth C. Rasmussen: Acetylene and Its Polymers. 150+ Years of History. Springer, ISBN 978-3-319-95488-2 , p. 9 f. ( limited preview in Google Book search).
- ↑ Entry on dichloroethane. In: Römpp Online . Georg Thieme Verlag, accessed on June 12, 2014.
- ^ A b Pieter Imhof (ed.), Jan Cornelis van der Waal (ed.): Catalytic Process Development for Renewable Materials. Wiley-VCH Verlag, 2013, ISBN 978-3-527-33169-7 , pp. 25-27.
- ↑ Manfred Baerns, Arno Behr, Axel Brehm, Jürgen Gmehling, Kai-Olaf Hinrichsen, Hanns Hofmann, Regina Palkovits, Ulfert Onken, Albert Renken: Technische Chemie . 2nd Edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 2013, ISBN 978-3-527-33072-0 , p. 551 .
- ↑ Oliver Konze: Pipeline filled for two weeks. Message in the Donaukurier from September 13, 2012.
- ↑ WH Keesom, KW Taconis: An x-ray goniometer for the investigation of the crystal structure of solidified gas. In: Physica. 2, 1935, p. 463, doi: 10.1016 / S0031-8914 (35) 90116-1 .
- ^ Karl Ziegler - Consequences and development of an invention. (PDF; 633 kB) Retrieved May 29, 2013 .
- ↑ Heike Kloppenburg, Thomas Gross, Martin Mezger, Claus Wrana: The elastic century. Synthetic rubbers. In: Chemistry in Our Time. 43, 2009, pp. 392-406, doi: 10.1002 / ciuz.200600515 .
- ^ Richard A. Love, Thomas F. Koetzle, Graheme JB Williams, Lawrence C. Andrews, Robert. Construction: Neutron diffraction study of the structure of Zeise's salt, KPtCl3 (C2H4) .H2O. In: Inorganic Chemistry. 14, 1975, p. 2653, doi: 10.1021 / ic50153a012 .
- ↑ Ethylene market study by Ceresana Research, December 2010
- ^ Sylvia Hughes: Antelope activate the acacia's alarm system. Retrieved June 9, 2015 .
- ↑ a b Schopfer, Peter. Brennicke, Axel. Mohr, Hans .: Plant Physiology . Spectrum Academic Publishing House, 2010, ISBN 978-3-8274-2351-1 .






![\ mathrm {n \ CH_ {2} {=} CH_ {2} \ longrightarrow [-CH_ {2} {-} CH_ {2} -] _ {n}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b8ab4bdba81d1fdcc16c8125cbb7a6528236dc45)





![\ mathrm {[PdCl_ {4}] ^ {2 -} + C_ {2} H_ {4} + H_ {2} O \ rightarrow CH_ {3} CHO + Pd + 2 \ HCl + 2 \ Cl ^ {-} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/84f36753ba6922fdba1d1d36fe0f86d94c664f66)
![\ mathrm {Pd + 2 \ CuCl_ {2} +2 \ Cl ^ {-} \ rightarrow [PdCl_ {4}] ^ {2 -} + 2 \ CuCl}](https://wikimedia.org/api/rest_v1/media/math/render/svg/572ed0fdcaf257aec043465677973e1a9651b67c)




