Serve
The dienes are a group of organic chemical compounds , the two carbon -Kohlenstoff- double bonds ( short contain C = C double bonds). The dienes are therefore among the alkenes or polyenes . The simplest dienes are the hydrocarbons propadiene , 1,3-butadiene and isoprene .
Classification and nomenclature
| Isomeric pentadienes | |
|---|---|
 conjugated diene 1,3-pentadiene |
|
 isolated diene 1,4-pentadiene |
|
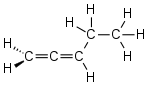 cumulative diene 1,2-pentadiene |
|
Serves differ in the arrangement of the double bonds, i.e. the constitution of the molecule:
- Are the carbon double bonds in a carbon chain separated by exactly one carbon single bond, such as. B. in the case of 1,3-pentadiene , one speaks of conjugated double bonds (Latin conjugare : to connect to a pair). Interactions (conjugation) can take place between the double bonds, through which the single bond in between can acquire a “partial double bond character”. However, it must be taken into account that the length of the = C – C = bond is smaller than with alkanes (sp 2 -sp 2 bond) even without “conjugation” . Such compounds are sometimes referred to as conjuens .
- In the case of isolated double bonds , the double bonds are separated from one another by at least two CC single bonds , e.g. B. at 1,4-pentadiene . As a rule, the double bonds do not interact with one another.
- With cumulative double bonds (Latin cumulatus : heaped, cumulus : heap), the two carbon double bonds are not interrupted by other bonds, such as B. with 1,2-pentadiene . The carbon atoms are arranged linearly along the cumulative double bonds, since the central carbon atoms are in sp hybridization . Compounds with two accumulated double bonds are called allenes .
The name of a diene, like that of an alkene, is derived from that of the corresponding alkane , with the ending -an being replaced by the Greek numeral ( di for two ) representing the number of double bonds and the ending -en characterizing the alkenes . The positions of the double bonds are indicated by prefixed Arabic numbers separated by commas. The numbering of the bonds is done in such a way that the smallest possible numbers can be used in the name of the alkene. The numbers are linked to the name with a hyphen.
Example: butane (alkane) → 1,2-butadiene (alkene / diene, two double bonds at position 1 and 2 in the carbon chain)
Those dienes that contain no other heteroatoms and are only hydrocarbons are called alkadienes .
properties
structure
The bond length of the single and double bonds in dienes differs from those in alkanes or alkenes such as ethene . CC single bonds are usually 0.154 nm in length . CC double bonds are 0.134 nm long. However, CC single bonds that follow a CC double bond are shorter than 0.1526 nm.
This is because, due to the sp² hybridization of the carbon atom that is involved in the single and double bond, the binding molecular orbitals do not have the same spatial extent as in sp³ hybridization, for example, in the case of alkanes.
Conformation
Dienes have CC single bonds around which parts of the molecule can rotate. This enables different, energetically different states ( conformations ) of the molecule.
The rotation around the single bonds results in conformations in which two double bonds are on the same side as seen from the single bond or on opposite sides. One then speaks of cisoid or transoid double bonds.
configuration
Due to the CC double bonds occurring in the molecule, the phenomenon of cis-trans isomerism occurs in polyenes . Two different spatial orientations ( configurations ) of the carbon chain are possible at both double bonds . Since these configurations can only be converted into one another by breaking and reestablishing bonds , in this case the double bond, this results in at least physically different connections.
Mesomerism
In the case of conjugated dienes, the mesomerism also has an influence on the bond lengths and relationships.

1. CC double bonds between carbon atoms 1 and 2 or 3 and 4;
2. CC double bond between carbon atoms 2 and 3;
3. Resonance hybrid orbital from states 1. and 2.
There are two mesomeric boundary structures of 1,3-butadiene, which are characterized by different electron distributions ( see figure above). All carbon atoms are sp² hybridized . Namely, each carbon atom of a perpendicular to the molecular plane standing p. Z - atomic orbital has obtained by overlapping with a p z a orbital of an adjacent carbon atom double bond (more specifically, a can bond) form. But this is also possible with more distant carbon atoms, but less likely.
Hence, one can formulate two structures for 1,3-butadiene:
- Overlapping of the p z orbitals of carbon atoms 1 and 2 or 3 and 4 and formation of the corresponding double bonds.
- A central double bond is formed by overlapping the p z orbitals of carbon atoms 2 and 3. The p z orbitals of carbon atoms 1 and 4 can also interact with one another. However, from a spatial point of view, this is very unlikely, so that 1,3-butadiene is only present in small proportions in this mesomeric structure.
Neither structure 1 nor 2 can, on their own, represent the actual bonding relationships. By superimposing these two structures, a so-called resonance hybrid for 1,3-butadiene is obtained, a hybrid of the two mesomeric limiting forms 1 and 2. This shows a ( delocalized ) electron density distributed over the entire molecule .
On the basis of these considerations, it can be said in summary that the double bonds in 1,3-butadiene to a certain extent have the properties of single bonds and, conversely, single bonds also have a slight double bond character.
stability
The so-called heat of hydrogenation is a relative measure of the stability of hydrocarbons with multiple bonds such as dienes . This is the energy that is released when hydrogen to the double bonds is added is ( hydrogenation ). It is observed that the hydrogenation of conjugated dienes releases less energy than non-conjugated dienes.
Reactions
Serves are accessible for additions. In particular, they enter into Diels-Alder reactions .
Manufacturing
Cracking of alkanes
The simplest conjugated diene 1,3-butadiene is obtained by catalytic cracking of n -butane . This dehydration produces butene , propene and methane as by-products .
Dehydration of diols
Acid-catalyzed dehydration of diols can be used to prepare conjugated dienes.
Dimerization of Alkynes
Ethyne reacts in the presence of ammonium chloride and copper (I) chloride with dimerization to form vinyl acetylene . Then 1,3-butadiene is produced by hydrogenation .
Individual evidence
- ^ Siegfried Hauptmann : Organische Chemie , 2nd edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 245.
- ↑ a b Joachim Buddrus: Fundamentals of organic chemistry . Walter de Gruyter, 2011, ISBN 3-11-024894-8 , p. 368 ( limited preview in Google Book search).

