Delocalization
In chemistry, we speak of delocalization (often also π-electron system ) when one or more electrons in an atom group, i.e. a molecule or molecular ion , cannot be precisely localized, but rather is distributed over the individual atoms. Mesomeric boundary structures are used to describe this charge distribution according to the VB theory .
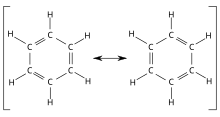
A special case of delocalized charges can be found in conjugated systems of aromatic compounds . Their π electrons are not located in isolated π orbitals belonging to two carbon atoms , but belong to molecular orbitals that extend over several carbon atoms; the π electrons are distributed in an “electron cloud” over the carbon atoms of the entire aromatic system. A classic example of such an aromatic molecule with delocalized π electrons is benzene . The ring structure of benzene was correctly postulated by Kekulé in 1865 (French article) (German article 1866) and the boundary structures in 1872 .
Molecules with increasingly large delocalized π-electron systems show absorption bands that migrate from the UV range to the visible. The reason for this is the ever smaller energetic distance between the ground and excited states, which can already be rationalized with the very simplified model of the particle in the box .
In addition to z. B. polynuclear, fused aromatics, this also occurs in linear π-electron systems such as carotenoids . Here, too, the individual π bonds overlap to form a conjugated system; this is also referred to as partial delocalization.
Other examples of such colored π-electron systems are charge transfer complexes such as potassium permanganate or sandwich complexes such as ferrocene and titanocene .
Individual evidence
- ↑ Entry on delocalization . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.D01583 .
- ↑ Aug. Kekulé: Sur la constitution des substances aromatiques . In: Bulletin de la Societe Chimique de Paris . 3, No. 2, 1865, pp. 98-110.
- ↑ Aug. Kekulé: Studies on aromatic compounds . In: Annals of Chemistry and Pharmacy . 137, No. 2, 1866, pp. 129-196. doi : 10.1002 / jlac.18661370202 .
- ↑ August Kekulé: About some condensation products of the aldehyde , Liebigs Ann. Chem. 1872 , 162 (1), pp. 77-124; doi : 10.1002 / jlac.18721620110 .