Butenine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
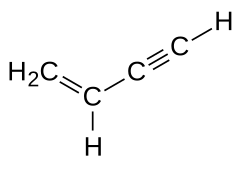
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Butenine | |||||||||||||||
| other names | ||||||||||||||||
| Molecular formula | C 4 H 4 | |||||||||||||||
| Brief description |
unstable, flammable gas |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 52.07 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
0.71 g l −1 (0 ° C, 1013 hPa) |
|||||||||||||||
| Melting point |
−92 ° C |
|||||||||||||||
| boiling point |
5 ° C |
|||||||||||||||
| Vapor pressure |
250 k Pa (30 ° C) |
|||||||||||||||
| Refractive index |
1.4161 (1 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Butenine , often also referred to as vinyl acetylene , is a colorless gas. It is an organic-chemical compound and is the smallest possible representative of the enynes , a group of polyunsaturated hydrocarbons that structurally have both alkene and alkyne character.
Manufacturing
In the laboratory it is most easily accessible by double dehydrohalogenation ( elimination ) of 1,3-dichloro-2-butene . Technically, butenine can be produced by the dimerization of ethyne (acetylene). The dimerization is promoted by the use of a Nieuwland catalyst ( copper (I) chloride ) in the aqueous phase. Butenin was first produced in large quantities by employees of the DuPont company in 1931.
properties
As a highly unsaturated compound, vinyl acetylene tends to break down spontaneously. It is not stable in storage and cannot be purchased in pure form. For safety reasons, it should only be handled diluted with inert gases. Even if it is not yet officially labeled as hazardous, it is - like any gaseous hydrocarbon - to be regarded as extremely flammable. Its mixtures with air are explosive in certain concentration limits.
Other physical data
The critical temperature is 183 ° C.
use
Vinylacetylene was previously used as an intermediate in the synthesis of chloroprene (2-chloro-1,3-butadiene). The chloroprene was carried addition of hydrogen chloride produced at Butenin.
Vinyl acetylene can be polymerized to polyvinyl acetylene in a controlled manner , which is suitable, for example, as a starting material for carbon fibers .
Individual evidence
- ↑ a b c d e Entry on Butenin in the GESTIS substance database of the IFA , accessed on February 27, 2017(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-74.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ GF Hennion, Charles C. Price, and Thomas F. McKeon, Jr .: Monovinylacetylene In: Organic Syntheses . 38, 1958, p. 70, doi : 10.15227 / orgsyn.038.0070 ; Coll. Vol. 4, 1963, p. 683 ( PDF ).
- ↑ Klaus Weissermel , Hans-Jürgen Arpe Industrial organic chemistry: Important preliminary and intermediate products. Wiley-VCH, Weinheim 2007, ISBN 3-527-31540-3 , p. 132.
- ↑ JA Nieuwland et al: Acetylene Polymers and Their Derivatives. I. The Controlled Polymerization of Acetylenes. In: Journal of the American Chemical Society 53/1932, pp. 4197-4202; doi: 10.1021 / ja01362a041 .
- ^ WH Carothers et al: Acetylene Polymers and their Derivatives. II. A New Synthetic Rubber: Chloroprene and its Polymers In: Journal of the American Chemical Society 53/1937, pp. 4203-4225.
- ↑ SG Grigoryan et al .: Polymerization of vinylacetylene compounds under the action of palladium salt in conditions of homogeneous catalysis. In: Polymer Science USSR 27/1985, pp. 1886-1890.
- ↑ A. Mavinkurve et al: An initial evaluation of poly (vinylacetylene) as a carbon fiber precursor. In: Carbon 33/1995, pp. 757-761.
literature
- H. Siegel et al: Alkynes and Cumulenes, XVII. Photoadditions of vinyl acetylene to other unsaturated hydrocarbons. In: Chemischeberichte 118/1984, pp. 597-612; doi: 10.1002 / cber.19851180219 .
- HW Morgan, JW Goldstein: The Microwave Spectrum of Vinylacetylene. In: J. Chem. Phys. 20/1952, p. 1981; doi: 10.1063 / 1.1700372 .
- A. Fahr, A. Nayak: Temperature dependent ultraviolet absorption cross sections of propylene, methylacetylene and vinylacetylene. In: Chemical Physics 203/1996, pp. 351-358; doi: 10.1016 / 0301-0104 (95) 00401-7 .
- T. Bally, Weilin Tang Martin Jungs : The electronic structure of the radical cations of butadiene, vinylacetylene and diacetylene: similarities and differences. In: Chemical Physics Letters 190/1992, pp. 453-459; doi: 10.1016 / 0009-2614 (92) 85172-7 .
