Enine
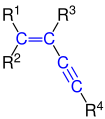
The enynes are a group of substances that contain a C = C double bond and a C≡C triple bond . The name is derived from the suffixes -en (from alkene ) and -in (from alkyne ).
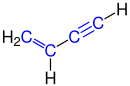
The simplest representative of this group is butenine (vinylacetylene).
Classification
Depending on the position of the double and triple bond, a distinction is made between isolated and conjugated enynes. The enynes form a subgroup of the enynes .
Occurrence and use
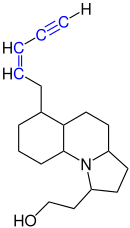
Enynes can be produced synthetically, but they also occur in nature. For example, gephyrotoxin is found in harlequin tree climbers .
Enynes are used in organic chemistry, for example in enyne metathesis for the production of 1,3- dienes . Furthermore, allenes can be prepared via enynes in a conjugate 1,6-addition .
See also
Commons : Enynes - collection of images, videos and audio files
Commons : Enyne metathesis - collection of images, videos and audio files
Individual evidence
- ↑ Marco Santarem, Corinne Vanucci-Bacqué & Gérard Lhommet: Formal Total Synthesis of (+) - Gephyrotoxin. In: The Journal of Organic Chemistry , Vol. 73 (16), pp. 6466-6469. doi : 10.1021 / jo801150e .
- ↑ Steven T. Diver & Anthony J. Giessert: Enyne Metathesis (Enyne Bond Reorganization). In: Chemical Reviews 2004, Vol. 104 (3), pp. 1317-1382. doi : 10.1021 / cr020009e .
- ↑ N. Krause, A. Gerold: Regio- and stereoselective syntheses with organocopper reagents. In: Angewandte Chemie 1997, Vol. 109 (3), pp. 194-213. doi : 10.1002 / anie.19971090304 .