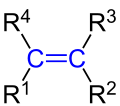Alkenes
| Alkenes |
|
General structural formula for alkenes with the characteristic C = C double bond between two sp 2 -hybridized carbon atoms ( marked in blue ). The following applies: R 1 to R 4 are hydrogen atoms or alkyl radicals. In dienes, one of the radicals R 1 to R 4 is an alkenyl group . |
| Ethene - also called ethylene - is the simplest alkene. |
| Propene, often called propylene. |
| 1-butene or 1-butene |
| Isobutene, correct IUPAC name: 2-methylpropene. |
| cis -2-butene or ( Z ) -2-butene |
| trans -2-butene or ( E ) -2-butene |
Alkenes (formerly also olefins ) are chemical compounds from the group of aliphatic hydrocarbons that have a carbon-carbon double bond in any position in the molecule. Alkenes are unsaturated compounds in contrast to the alkanes , in which all valences of the carbon atom are covered ( saturated ). Alkenes occur on a small scale in petroleum , in nature they are used as pheromones and phytohormones . They are the most important basic products in industrial organic chemistry.
The alkenes form a homologous series with the general empirical formula C n H 2n beginning with ethene . The outdated name olefins derives from the old name olefin of ethene, as it forms oily, water-insoluble liquids with halogens, which consist of haloalkanes . There are also cyclic alkenes, the cycloalkenes , the most important representative of which is cyclohexene .
properties
The alkenes from ethene (C 2 H 4 ) to butene (C 4 H 8 ) are gaseous and thus highly volatile. From pentene with 5 to pentadecene with 15 carbon atoms, the alkenes are liquid. Alkenes with more than 15 carbon atoms are solid (in each case under standard conditions ). Alkenes are sparingly soluble in water; they burn with a sooty flame. The alkenes are reactive. The weak double bond offers a point of attack for reagents, more precisely it is the π bond that is attacked electrophilically. Alkenes react with halogens to form dihaloalkanes. This happens through an electrophilic addition.
Here are the most important alkenes from ethene (C 2 H 4 ) to decene (C 10 H 20 ) with names and empirical formulas:
- Ethene : C 2 H 4
- Propene : C 3 H 6
- Butene : C 4 H 8
- Pentene : C 5 H 10
- Witches : C 6 H 12
- Hepten : C 7 H 14
- Octene : C 8 H 16
- Nons : C 9 H 18
- Decene : C 10 H 20
There are several structural isomers of butene and the higher alkenes. The general empirical formula of the unsubstituted alkenes is: C n H 2n .
Nomenclature and isomers
General nomenclature
In general, alkenes are named according to IUPAC in the same way as alkanes, with the suffix -an being replaced by -en .
The position of the double bond in the carbon chain is indicated in the name by a number that denotes the carbon atom where the double bond begins. It counts as a functional group and has to be taken into account for the order of the numbering, i.e. have the smallest possible number. For molecules with several functional groups, the number is placed directly in front of the -en , otherwise also in front of the name. Multiple double bonds are preceded by the corresponding Greek numeral.
| ( EZ ) nomenclature for alkenes. The CIP priority of the four substituents is a > b and c > d . |
In addition to the structural isomerism , in which the carbon atoms are arranged differently, the cis - trans isomerism can also occur in alkenes at the C = C double bond .
Since the double bond, in contrast to the single bond, cannot rotate freely, there can be two possible arrangements when atoms or groups of atoms are attached to the double bond. Cis-trans isomers differ in their physical and chemical properties. They can be distinguished by the dipole moment and by IR spectroscopy . While the cis is mentioned in the connection name, the trans can also be omitted.
The cis - trans isomerism can be traced back to the example of the isomeric but-2-enes . In the cis -but-2-ene, both methyl groups lie as chain residues on this side (Latin: cis ), i.e. on the same side. In the case of trans -but-2-ene, the methyl groups are on the other (Latin: trans ) side of the double bond.
The IUPAC replaced the cis / trans designation (since it is easily misleading if there are more than two substituents; just look at ( E ) -2-bromo-1-chloro-1-fluoro-ethene!) By E / Z , where ( e ) ( e ntgegengesetzt) usually - but not always - for trans stands and ( Z ) ( z ogether) for cis . The mutual position of the substituents with the highest CIP priority is given. For more detailed information see ( E , Z ) -isomerism .
Traditionally, some simple substances with ( E , Z ) -isomerism have different names: fumaric acid [( E ) -butenedioic acid] and maleic acid [( Z ) -butenedioic acid] and their derivatives are examples of this.
Serves and polyenes
Compounds with two double bonds are called dienes , and those with three double bonds are called trienes . These are called polyenes in general . The naming of the molecules follows the same rules as for the monounsaturated alkenes, see 1,3-butadiene and isoprene . The number of possible cis - trans isomers increases drastically because there are cis - trans isomers for each of the double bonds .
use
Due to the very reactive double bond, alkenes are important starting materials for many other basic materials in the chemical industry.
Alkenes are used as fuels and for the production of halogenated hydrocarbons , alcohols , ketones , glycols , olefin oxides, plastics and detergent components . Propene is used for the synthesis of e.g. B. glycerine , phenol , isopropyl alcohol , epoxy resins and required for the polymerization of polypropylene .
production method
Manufacturing process in general
Alkenes can be prepared by various methods. One possibility is the pyrolytic dehydrogenation and cleavage of alkanes ( cracking ). The short-chain alkanes are split into alkenes and hydrogen at 450–500 ° C in the presence of mixed oxide catalysts. In the case of higher alkanes, however, this process does not make much sense since it can produce many different isomers , the separation of which is very laborious, if not impossible.
Another possibility to produce alkenes is the partial hydrogenation of alkynes . Alkynes are hydrogenated in the presence of the Lindlar catalyst . This slightly poisoned catalyst prevents further hydrogenation of alkenes to alkanes . Because the hydrogen molecule approaches the triple bond from one side, only ( Z ) -alkenes are formed.
- Ethyne is hydrogenated to ethene by taking up one equivalent of hydrogen.
β eliminations
In general, compounds with the structural fragment CH – CX can be converted into an alkene with the structural fragment C = C by splitting off HX. In the educt with the structure fragment CH-CX, the H and the group X are bound to directly adjacent carbon atoms.
Dehydration
The dehydration of alcohols takes place in an acidic environment. Water can be eliminated much more easily from tertiary alcohols than from secondary or even primary alcohols.
Alcohols can also be dehydrated to alkenes at elevated temperatures (e.g. approx. 200 ... 250 ° C) using porous catalysts with a high internal surface area (such as aluminum oxide Al 2 O 3 ). Secondary alcohols of the type CH 3 –CHOH – CH 2 –R (R = alkyl radical ) result in a mixture of 1-olefins, cis-2- and trans-2-olefins:
Dehydrohalogenation
Analogously, haloalkanes can be converted into alkenes. In contrast to the dehydration of alcohols, this reaction, known as dehydrohalogenation, takes place under basic conditions. Here, too, tertiary hydrogen halides can be dehydrohalogenated more easily than secondary ones, and these in turn more easily than primary hydrogen halides.
- Chlorethane is dehydrohalogenated to ethene and hydrochloric acid.
Saytzeff rule
Regioselectivity can be observed in these β-eliminations. Several products can be created under certain circumstances. The Saytzeff rule applies :
In addition to the OH group or the halogen atom, the hydrogen is eliminated from the neighboring C atom which has the fewest hydrogen atoms. In other words, the most highly substituted alkene is formed.

Dehalogenation
The third possible β-elimination is the dehalogenation of 1,2-dihaloalkanes. In alcohols, in the presence of zinc, two halogen atoms of the same type are eliminated from neighboring carbon atoms. This creates the alkene and the halogen in molecular form:
- 1,2-Dibromoethane is dehalogenated to ethene and bromine.
Dehydration
Alkenes can be obtained from alkanes by partial dehydrogenation . To this end, hydrogen is eliminated from the corresponding alkane. The number of carbon atoms remains the same.
Pyrolysis of quaternary ammonium hydroxides
Alkenes are formed in the pyrolysis of quaternary ammonium hydroxides if at least one alkane group on the nitrogen has 2 or more carbon atoms. The implementation is as follows:
Typical reactions
The reaction mechanism typical for alkenes is electrophilic addition . The typical detection method for alkenes, the bromine water test, is based on this reaction : alkenes or other unsaturated hydrocarbons are combined with brown-colored bromine water, bromine being added to the alkene and the mixture rapidly decolorizing.
Addition of hydrogen halides
The addition of hydrogen halides is similar to the addition of halogens such as bromine. Although theoretically different reaction products are conceivable in the addition to unsymmetrical alkenes, depending on which carbon atom involved in the double bond the halogen atom is attached to, the addition reaction preferably proceeds regioselectively according to Markovnikov's rule .
A regioselective reaction is a chemical change that preferably occurs at one of several possible locations. The most stable carbenium ion is always formed as an intermediate product through reaction with the proton H + of the hydrohalic acid. Mesomerism effects are always more important than inductive effects for this consideration. The halide then adds to the C + of the carbenium ion. If the mesomerism stabilization of the carbenium ion is not possible, the Markovnikov rule can be applied: In the case of asymmetrical alkenes, the electrophilic addition of hydrogen halides takes place in such a way that the halogen binds preferentially to the carbon atom that has the fewest hydrogen atoms; the hydrogen atom on the other hand to the carbon atom richest in hydrogen. The reason for this is that alkyl groups act as electron donors ( + I effect and hyperconjugation ) and thereby favor the distribution (delocalization) of the positive charge. The stability of the positive carbenium ions is greater, the more alkyl groups are bound to the charged carbon atom. Therefore a tertiary carbenium ion is more stable than a secondary and primary. The same also applies to radicals (see deviation # 2), since these also suffer from a lack of electrons.
Deviations from Markovnikov's rule occur, among other things, with radical addition and hydroboration . These reactions produce the anti-Markovnikov product:
- In hydroboration, the boron atom (electrophile) is added to the negative partially charged carbon atom (lower substituted) of the double bond, whereas the hydrogen atom (nucleophile) is added to the positively partially charged carbon atom (higher substituted). Since the hydrogen atom is mostly the electrophile, this reversal of roles breaks the rule.
- In the radical addition of z. B. Hydrogen bromide does not attack the double bond of the electrophile (hydrogen), but a bromine radical the double bond. The bromine atom binds to the less substituted carbon atom so that the radical is formed on the more highly substituted one. The lack of electrons on the radical can be stabilized by hyperconjugation with the substituents and by the + I effect. Only then does this radical attack another H-Br molecule and thus acquire its hydrogen atom, which is then located on the more highly substituted carbon atom and thus violates the rule.
Reaction with concentrated sulfuric acid
The deposition takes place according to the Markovnikov rule , unless mesomeric effects dominate. Technically, this process is used to prepare alcohols from alkenes. Alkyl sulfuric acid can easily be converted into alcohols by hydrolysis .
Reaction with hypochlorous acid
This reaction is regioselective. The chlorine atom attaches to the carbon atom, which has the most hydrogen atoms if no mesomeric effects occur. The reaction with nitrosyl chloride and nitrosyl bromide to alkenes also takes place with addition.
Reaction with an oxidizing agent
The oxidation takes place either with osmium tetroxide or with alkaline potassium permanganate solution . First, a cyclic ester is formed with a cis addition, the hydrolysis leads to a cis - 1,2-diol .
Ozonation of C = C double bonds
When ozone is passed into anhydrous alkenes, ozonides are formed , which are explosive when dry. The ozonolysis cleaves the double bond completely and thus to determine the structure of a carbon chain an important reaction because the cleavage products to provide information on the location of the double bond. The ozone can be generated by discharge from atmospheric oxygen in an ozonizer.
Catalytic hydrogenation
The hydrogenation is the addition of hydrogen to the double bond, which thereby becomes a single bond, with the aid of a catalyst . The reaction takes place at room temperature in the presence of platinum or palladium , which is saturated with hydrogen.
Example:
Conversion of cis - to trans - alkenes and vice versa
( E ) -Alkenes [example: ( E ) -stilbene , melting point 124 ° C. ] can be converted photochemically into ( Z ) -alkenes [example: ( Z ) -stilbene, melting point 1 ° C. ]. The reaction is reversible. - In rare exceptional cases (example: ranitidine ) the energy barrier for the conversion of specially substituted alkenes is so low at room temperature that the ( E ) form spontaneously converts to the ( Z ) form and vice versa. In other words: ( E ) isomer and ( Z ) isomer can be in equilibrium with one another in rare exceptional cases.
proof
An alkene (e.g. ethene) is introduced into brown bromine water for unspecific detection of the double bond, especially to distinguish it from alkanes . The reaction can also be carried out without adding energy, e.g. B. light, expire. The alkene molecule adds a bromine atom to each carbon atom of the C = C double bond according to the reaction mechanism of electrophilic addition ; the corresponding haloalkane is formed as the reaction product . For example:
- Bromine and ethene react to form 1,2-dibromoethane
The bromine water is discolored due to this reaction; an alkane introduced would not discolour the brown bromine water. However, phenols and many reducing compounds also discolor a bromine solution.
The so-called Baeyer sample is used for the general detection of C = C double bonds or alkenes. The alkene is introduced into a potassium permanganate solution in a weakly alkaline or acidic medium, whereupon the solution turns brown or becomes colorless. Alcohol and manganese dioxide or manganese (II) ions are formed.
literature
- Allinger , Cava , de Jongh , Johnson , Lebel , Stevens : Organic Chemistry , 1st Edition, Walter de Gruyter, Berlin 1980, ISBN 3-11-004594-X , pp. 222-240.
- Beyer / Walter : Textbook of Organic Chemistry , 19th edition, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , pp. 62-76.
- Morrison / Boyd : Textbook of Organic Chemistry , 3rd Edition, Verlag Chemie, Weinheim 1986, ISBN 3-527-26067-6 , pp. 305-370.
- Streitwieser / Heathcock : Organic Chemistry , 1st Edition, Verlag Chemie, Weinheim 1980, ISBN 3-527-25810-8 , pp. 305-361.
- K. Peter C. Vollhardt , Neil E. Schore : Organic Chemistry , 4th Edition, Wiley-VCH, Weinheim 2005, ISBN 978-3-527-31380-8 , pp. 509-560.
Web links
- Entry to alkenes . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00224 Version: 2.3.3.
Individual evidence
- ↑ Dirk Dautzenberg, Helmut Knözinger: Influence of Steric and Inductive Effects on Product Distribution in the Dehydration of Secondary Alcohols on Alumina , Journal of Catalysis 33, 142-144 (1974) doi : 10.1016 / 0021-9517 (74) 90254-1 .
- ↑ Dirk Dautzenberg, Helmut Knözinger: Inductive Effects and Product Distributions in the Dehydration of Secondary Alcohols on Alumina. Reply to the Comments by BH Davis, Journal of Catalysis 58, 496-497 (1979) doi : 10.1016 / 0021-9517 (79) 90288-4 .
- ^ LF Fieser and M. Fieser: Textbook of Organic Chemistry , 3rd edition, Verlag Chemie, Weinheim / Bergstr. 1957, p. 260.
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 283.