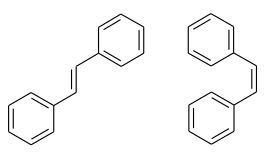
Stilbene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| trans (left) and cis isomer (right) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Stilbene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 14 H 12 | |||||||||||||||
| Brief description |
|
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 180.25 g mol −1 | |||||||||||||||
| Physical state |
|
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Stilbene ( 1,2-diphenylethene ) is an unsaturated hydrocarbon that can be seen as a symmetrical diphenyl derivative of ethene . There are two configurationally isomeric compounds with the cis and trans styles . The asymmetrically substituted diphenyl derivative of ethene is the constitutional or structural isomer 1,1-diphenylethene .
The name stilbene comes from the French chemist Auguste Laurent in 1845 and refers to the Greek word stilbein for shine, similar to the pearlescent mineral stilbit .
Presentation and extraction
The synthesis of trans -stilbene is possible by reacting benzaldehyde with diethyl benzylphosphonate in a Horner-Wadsworth-Emmons reaction . The alkylated phosphonic acid ester can be obtained by a Michaelis-Arbuzov reaction of diethyl phosphite with benzyl bromide .
An alternative production variant is the reaction of benzaldehyde with benzyl magnesium bromide. The industrial production of trans -stilbene takes place through a catalyzed oxidative dimerization of toluene or through a reductive dimerization of benzylidene chloride .
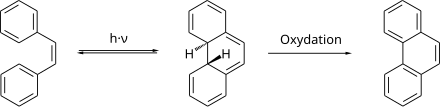
The production of cis -stilbene takes place through a photochemical isomerization of trans -stilbene. The isomerization reaction is reversible. Reisomerization to trans -stilbene is thermally possible.
The synthesis of pure cis -stilbene can also be achieved by metalating diphenylacetylene ( tolane ) with lithium and subsequent reaction with methanol at −78 ° C. Another production option is the decarboxylation of 2-phenylcinnamic acid.
properties
Physical Properties
trans -stilbene forms colorless, glossy crystals that melt at 125 ° C. The melting enthalpy is 27.37 kJ mol −1 , the melting entropy 68.8 J mol −1 K −1 . The molar heat capacity at 25 ° C has a value of 235.0 J · mol −1 · K −1 . A molar enthalpy of combustion of −7360.8 ± 3.9 kJ mol −1 and a molar enthalpy of formation of 136.7 kJ mol −1 were determined for the solid .
cis -stilbene is a colorless liquid which crystallizes to a solid below the melting point at 5.85 ° C. A boiling point of 135 ° C. was observed under a reduced pressure of 13 hPa. The enthalpy of vaporization is 66 ± 1 kJ mol −1 . A molar enthalpy of combustion of −7404.05 ± 0.75 kJ · mol −1 was determined for the liquid .
Chemical properties
cis -stilbene can be cyclized photochemically to 4a, 4b-dihydrophenanthrene. The cyclization product is thermodynamically unstable and easily reforms the cis stilbene. In the presence of oxidizing agents, in the simplest case of atmospheric oxygen, the thermodynamically stable phenanthrene is formed quickly .
The compound decomposes at higher temperatures. The specified decomposition temperature with the criterion of a decomposition rate of 1 mol% h −1 is 418 ° C.
use
trans -stilbene can be used as a monomer additive in copolymerizations . The crystals are suitable as scintillator materials . cis -stilbene is often used as an olefinic test substance to elucidate the mechanism and stereospecificity of organic syntheses. It can also be used as a starting substance in heterocycle synthesis and in cyclopropanation.
Stilbene derivatives
Occurrence
Stilbene derivatives are found in plants and are the product of two different biosynthetic pathways. One part comes from the shikimic acid route, the other from polyketide biosynthesis, a phenylpropanide building block being used as a starter unit for the iterative PKS synthesis of PKS III.
More stilbene derivatives are resveratrol , which is associated with health-promoting effects of red wine in combination, rhaponticin one from rhubarb isolable phytoestrogen , and pinosylvin , a in the heartwood of pine occurring 3,5-Stilbendiol.
use
A number of stilbene derivatives have hormone-like effects. The substance diethylstilbestrol (diethylstilbestrol, DES) was the first commercial oral estrogen preparation. Due to its anabolic effect, DES was used as a fattening aid in cattle and pig fattening until the early 1980s . However, DES is carcinogenic. That is why the use of stilbene and its derivatives in animals used for food production is prohibited in the EU. In Germany, this is regulated by law in the ordinance on substances with a pharmacological effect . In connection with hormone meat scandals, these stilbene derivatives were often referred to as “stilbenes” in media reports.
Other stilbene derivatives such as clomiphene , tamoxifen and toremifene are used as estrogen receptor modulators .
Stilbene derivatives are often used as optical brighteners , especially in textiles made from polymer materials , and as laser dyes . The special feature of stilbene compared to other aromatics and polyenes is exploited in that it fluoresces despite the double bond that characterizes the absorption . This is due to the strong change in the bond conditions in the excited state; In the excited S1 state, the double bond has a strong single bond character , whereas the single bonds to the rings have a double bond character. This greatly attenuates the torsional vibrations of the rings, which have a strong fluorescence-quenching character in other compounds of a similar structure, which results in the comparatively strong fluorescence quantum yield of stilbene.
literature
- Topics in Current Chemistry. Vol. 209. Springer, Berlin 2000, ISBN 3-540-66573-0 .
Web links
- Friedrich Katscher: The children of Stilböstrol. In: Wiener Zeitung . October 16, 1998 (accessed November 18, 2013).
Individual evidence
- ↑ Trans-stilbene data sheet (PDF) from Merck , accessed December 9, 2010.
- ↑ Data sheet cis-stilbene (PDF) from Merck , accessed on December 9, 2010.
- ↑ a b c d CRC Handbook of Chemistry and Physics. 87th edition. (CD-Rom version 2007), Taylor and Francis, Boca Raton (FL) 2007.
- ↑ a b c J. C. van Miltenburg, JA Bouwstra: Thermodynamic properties of trans-azobenzene and trans-stilbene. In: The Journal of Chemical Thermodynamics. 16, No. 1, 1984, pp. 61-65, doi : 10.1016 / 0021-9614 (84) 90075-2 .
- ^ A b S. Frisch, H. Hippler, J. Troe: UV Absorption Spectra and Formation Rates of Silibene in the High Temperature Kinetics of Benzyl Radicals. In: Journal of Physical Chemistry. 188, 1995, pp. 259-273, doi : 10.1524 / zpch.1995.188.Part_1_2.259 .
- ↑ a b c d e f g Entry on stilbenes. In: Römpp Online . Georg Thieme Verlag, accessed on April 17, 2014.
- ↑ a b Data sheet trans-stilbene, 96% from Sigma-Aldrich , accessed on February 26, 2013 ( PDF ).
- ↑ a b Organikum. 21st edition. Wiley-VCH 2001, ISBN 3-527-29985-8 .
- ↑ a b Römpp Lexikon Chemie. 10th edition, Georg Thieme, 1999.
- ^ H. Goerner, HJ Kuhn: Cis-Trans Photoisomerization of Stilbenes and Stilbene-Like Molecules , In: Advances in Photochemistry. 19, 1995, pp. 1-117, doi : 10.1002 / 9780470133507.ch1 .
- ↑ J. Saltiel, JT D'Agostino, ED Megarity, L. Metts, KR Neuberger, M. Wrighton, OC Zafiriow: Org. Photochem. 3, 1973, 1.
- ↑ FA Carey, RJ Sunberg: Advanced Organic Chemistry - Part A: Structure and Mechanisms. 5th edition. Springer, 2008, ISBN 978-0-387-68346-1 , pp. 1085-1091.
- ^ Carlo Bastianelli, Vincenzo Caia, Giampietro Cum, Raffaele Gallo, Vittorio Mancini: Thermal isomerization of photochemically synthesized (Z) -9-styrylacridines. An unusually high enthalpy of Z → E conversion for stilbene-like compounds. In: Journal of the Chemical Society, Perkin Transactions 2. No. 5, 1991, pp. 679-683, doi : 10.1039 / P29910000679 .
- ↑ G. Levin, Joseph Jagur-Grodzinski, Michael Szwarc: Simple and quantitative method of preparation of cis-stilbene and its deuterated analog, Ph-CD: CD-Ph. In: The Journal of Organic Chemistry. 35, No. 5, 1970, pp. 1702-1702, doi : 10.1021 / jo00830a109 .
- ↑ Sidney Marantz, George Thomson Armstrong: Heats of combustion of trans-stilbene and trans-2,2 ', 4,4', 6,6'-hexanitrostilbene. In: Journal of Chemical & Engineering Data. 13, No. 1, 1968, pp. 118-121, doi : 10.1021 / je60036a036 .
- ↑ Sidney Marantz, George T Armstrong: Corrections-Heats of Combustion of trans-Stilbene and trans-2,2 ', 4,4', 6,6'-Hexanitrostilbene (HNS). In: Journal of Chemical & Engineering Data. 13, No. 3, 1968, pp. 455-455, doi : 10.1021 / je60038a902 .
- ^ DS Brackman, PH Plesch: Some physical properties of cis-stilbene. In: Journal of the Chemical Society (Resumed). 1952, pp. 2188-2190, doi : 10.1039 / JR9520002188 .
- ^ Keith Yates, Robert S. McDonald: Thermomechanical probe into the mechanism of electrophilic addition to olefins. In: Journal of the American Chemical Society. 93, No. 23, 1971, pp. 6297-6299, doi : 10.1021 / ja00752a066 .
- ^ Frank B. Mallory, Clelia W. Mallory: Photochemistry of stilbenes. VI. Steric effects on the photocyclizations of some m-substituted stilbenes. In: Journal of the American Chemical Society. 94, No. 17, 1972, pp. 6041-6048, doi : 10.1021 / ja00772a017 .
- ↑ Longbin Liu, Bingwei Yang, Thomas J. Katz, Michael K. Poindexter: Improved methodology for photocyclization reactions. In: The Journal of Organic Chemistry. 56, No. 12, 1991, pp. 3769-3775, doi : 10.1021 / jo00012a005 .
- ↑ IB Johns, EA McElhill, JO Smith: Thermal Stability of Some Organic Compounds. In: Journal of Chemical & Engineering Data. 7, No. 2, 1962, pp. 277-281, doi : 10.1021 / je60013a036 .
- ↑ Text of the ordinance on substances with a pharmacological effect