Horner-Wadsworth-Emmons reaction
The Horner-Wadsworth-Emmons reaction (short: HWE reaction), sometimes - but incorrectly - referred to in textbooks as the Wittig-Horner reaction or Horner-Wittig reaction, is a chemical reaction with which ( E ) -alkenes are produced stereoselectively can be. For this purpose, aldehydes or ketones are reacted with the anions of organic phosphonates . In the reaction scheme, for. B. an aldehyde with a deprotonated phosphonate (Et = ethyl radical) converted to an α, β-unsaturated carboxylic acid ethyl ester:
In 1958 Leopold Horner published a modified Wittig reaction (after Georg Wittig ) using phosphonate-stabilized carbanions. William S. Wadsworth , later professor at South Dakota State University, and William D. Emmons (1924–2001), both chemists at the time at Rohm and Haas (Philadelphia), developed this reaction further.
In contrast to the phosphorylides of the Wittig reaction , the phosphonate-stabilized carbanions are more nucleophilic and more basic . Accordingly, unlike the phosphorylides, these can be alkylated. The by-product dialkyl phosphate salts can be easily removed by aqueous extraction.
Reaction mechanism
The exact mechanism of the Horner-Wadsworth-Emmons reaction is not yet known. It certainly begins with the deprotonation of the phosphonate , the phosphonate carbanion 1 is formed . It is also assumed that the nucleophilic addition of the carbanion to aldehyde 2 (or ketone), which leads to intermediate 3a or 3b , is the rate-determining step. If R 2 = H, the intermediates 3a and 4a and the intermediates 3b and 4b can convert into one another. An elimination of 4a and 4b gives the ( E ) -alkene 5 and the ( Z ) alkene 6 .
The ratio of the diastereomeric alkenes 5 and 6 does not depend on the stereochemical outcome of the carbanion addition, but largely depends on the extent of the chemical equilibrium between the intermediates ( 3a , 3b , 4a and 4b ).
The electron withdrawing group (in short: EWG - electron withdrawing group) in the α-position to the phosphonate is absolutely necessary for the reaction. In the absence of an “EWG”, the end product of the reaction is the α-H-hydroxyphosphonate 3a or 3b . These α-H-hydroxyphosphonates can be converted into alkenes with diisopropylcarbodiimide .
Stereoselectivity
The HWE reaction favors the formation of ( E ) -alkenes. In general, the better the equilibrium between the intermediates can be established, the higher the selectivity or the ( E ) -alkene content.
Disubstituted alkenes
SK Thompson and Clayton H. Heathcock published a systematic study of the reaction of trimethylphosphonoacetate with various aldehydes. While the individual differences were small, there was a cumulative effect that made it possible to control the stereoselectivity with the help of the structure of the phosphonate. The following conditions increase the E stereo selectivity:
- Increasing steric hindrance at the aldehyde
- Higher reaction temperatures
- For salts: Li > Na > K
- Solvent DME instead of THF
In another study it could be shown that sterically demanding phosphonates and “EWGs” also increase the ( E ) -alkene selectivity.
Trisubstituted Alkenes
The steric demand of the phosphonates and the EWGs actually shows an effect on the reaction of α-branched phosphonates with aliphatic aldehydes.
| R 1 | R 2 | Fraction of alkenes ( E : Z ) |
|---|---|---|
| methyl | methyl | 5:95 |
| methyl | Ethyl | 10:90 |
| Ethyl | Ethyl | 40:60 |
| Isopropyl | Ethyl | 90:10 |
| Isopropyl | Isopropyl | 95: 5 |
Aromatic aldehydes usually only give ( E ) -alkenes. If you want to prepare ( Z ) -alkenes from aromatic aldehydes, the Still-Gennari variant (see below) is one possibility.
Olefination of Ketones
The stereoselectivity of the HWE is low to average here.
Variations
Base-sensitive substrates
Since many substrates are sensitive to sodium hydride , some studies have been carried out on milder bases. On the one hand “Masamune and Roush” with lithium chloride and DBU and “Rathke” with lithium or magnesium halogens with triethylamine and other bases.
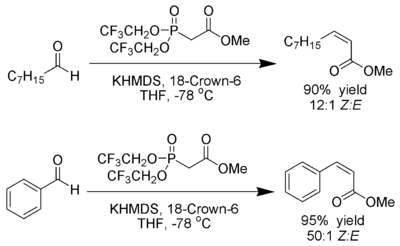
Still-Gennari variant
Still and Gennari developed conditions that allow a reaction with very high stereoselectivity to ( Z ) -alkenes. Phosphonates with electron-withdrawing groups (e.g. trifluoroethyl) are used together with strongly dissociating conditions ( KHMDS and [18] -crown-6 in THF ).
Ando has postulated that the use of electron-poor phosphonates accelerates the elimination of the oxophosphonates.
See also
- Michaelis-Arbuzov reaction for obtaining the reagents for the HWE reaction
- Peterson olefination
- Tebbe methylenation
literature
- William S. Wadsworth: Synthetic Applications of Phosphoryl-Stabilized Anions. In: Organic Reactions. 25, No. 2, 1977, pp. 73-253, doi: 10.1002 / 0471264180.or025.02 .
- John Boutagy, Richard Thomas: Olefin synthesis with organic phosphonate carbanions. In: Chemical Reviews. 74, No. 1, 1974, pp. 87-99, doi: 10.1021 / cr60287a005 .
- Sarah E. Kelly: Alkene Synthesis. In: Ian Fleming, Barry M. Trost (Eds.): Comprehensive Organic Synthesis. Volume 1: Additions to CX π-Bonds, Part 1, Pergamon, Oxford 1991, ISBN 0-08-040592-4 , pp. 729-817.
- Bruce E. Maryanoff, Allen B. Reitz: The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. In: Chemical Reviews. 89, No. 4, 1989, pp. 863-927, doi: 10.1021 / cr00094a007 .
Individual evidence
- ^ Leopold Horner, Hellmut Hoffmann, Hans G. Wippel: Organophosphorus compounds, XIII. Preparation of phosphinic acids from phosphine oxides. In: Chemical Reports. 91, No. 1, 1958, pp. 64-67, doi: 10.1002 / cber.19580910114 .
- ^ Leopold Horner, Hellmut Hoffmann, Hans G. Wippel, Günther Klahre: Organophosphorus compounds, XX. Phosphine oxides as olefination reagents. In: Chemical Reports. 92, No. 10, 1959, pp. 2499-2505, doi: 10.1002 / cber.19590921017 .
- ^ William S. Wadsworth, William D. Emmons: The Utility of Phosphonate Carbanions in Olefin Synthesis. In: Journal of the American Chemical Society. 83, No. 7, 1961, pp. 1733-1738, doi: 10.1021 / ja01468a042 ( Science Citation Classics, pdf ).
- ^ WS Wadsworth, Jr., WD Emmons: Ethyl Cyclohexylideneacetate In: Organic Syntheses . 45, 1965, p. 44, doi : 10.15227 / orgsyn.045.0044 ; Coll. Vol. 5, 1973, p. 547 ( PDF ).
- ^ Rolf Olaf Larsen, Gunnar Aksnes: Kinetic Study of the Horner Reaction. I . In: Phosphorus and Sulfur and the Related Elements . tape 15 , no. 2 , April 1983, p. 219-228 , doi : 10.1080 / 03086648308073297 .
- ↑ Gerard Lefebvre, Jacqueline Seyden-Penne: The mechanism of the Horner-Emmons modification of the Wittig reaction. In: Journal of the Chemical Society D: Chemical Communications. , No. 20, 1970, pp. 1308-1309, doi: 10.1039 / C29700001308 .
- ↑ EJ Corey , George T. Kwiatkowski: The Synthesis of Olefins from O, O'-Dialkyl β-Lithioalkylphosphonothioate Esters. In: Journal of the American Chemical Society. 88, No. 23, 1966, pp. 5654-5656, doi: 10.1021 / ja00975a057 .
- ↑ John F. Reichwein, Brian L. Pagenkopf: A New Horner-Wadsworth-Emmons Type Coupling Reaction between Nonstabilized β-Hydroxy Phosphonates and Aldehydes or Ketones. In: Journal of the American Chemical Society. 125, No. 7, 2003, pp. 1821-1824, doi: 10.1021 / ja027658s .
- ^ Scott K. Thompson, Clayton H. Heathcock: Effect of cation, temperature, and solvent on the stereoselectivity of the Horner-Emmons reaction of trimethyl phosphonoacetate with aldehydes. In: The Journal of Organic Chemistry. 55, No. 10, 1990, pp. 3386-3388, doi: 10.1021 / jo00297a076 .
- ↑ Hiroto Nagaoka, Yoshito Kishi: Further synthetic studies on rifamycin s. In: Tetrahedron. 37, No. 23, 1981, pp. 3873-3888, doi: 10.1016 / S0040-4020 (01) 93261-2 .
- ↑ Mary A. Blanchette, William Choy, Jeffery T. Davis, Amy P. Essenfeld, Satoru Masamune, William R. Roush, Toshiya Sakai: Horner-wadsworth-emmons reaction: Use of lithium chloride and an amine for base-sensitive compounds. In: Tetrahedron Letters. 25, No. 21, 1984, pp. 2183-2186, doi: 10.1016 / S0040-4039 (01) 80205-7 .
- ↑ Michael W. Rathke, Michael Nowak: The Horner-Wadsworth-Emmons modification of the Wittig reaction using triethylamine and lithium or magnesium salts. In: The Journal of Organic Chemistry. 50, No. 15, 1985, pp. 2624-2626, doi: 10.1021 / jo00215a004 .
- ^ Ian Paterson, Cape Sun Yeung, Jeff B. Smaill: The Horner-Wadsworth-Emmons Reaction in Natural Products Synthesis: Expedient Construction of Complex (E) -Enones Using Barium Hydroxide. In: Synlett. 1993, No. 10, 1993, pp. 774-776, doi: 10.1055 / s-1993-22605 .
- ^ Daniele Simoni, Marcello Rossi, Riccardo Rondanin, Angelica Mazzali, Riccardo Baruchello, Cinzia Malagutti, Marinella Roberti, Francesco Paolo Invidiata: Strong Bicyclic Guanidine Base-Promoted Wittig and Horner − Wadsworth − Emmons Reactions. In: Organic Letters. 2, No. 24, 2000, pp. 3765-3768, doi: 10.1021 / ol0001665 .
- ↑ Landy K. Blasdel, Andrew G. Myers: Use of Lithium Hexafluoroisopropoxide as a Mild Base for Horner-Wadsworth-Emmons Olefination of Epimerizable Aldehydes. In: Organic Letters. 7, No. 19, 2005, pp. 4281-4283, doi: 10.1021 / ol051785m .
- ↑ W. Clark Still, Cesare Gennari: Direct synthesis of Z-unsaturated esters. A useful modification of the horner-emmons olefination. In: Tetrahedron Letters. 24, No. 41, 1983, pp. 4405-4408, doi: 10.1016 / S0040-4039 (00) 85909-2 .
- ↑ C. Patois, P. Savignac, E. About-Jaudet, N. Collignon ,: Bis (trifluoroethyl) (carboethoxymethyl) phosphonate In: Organic Syntheses . 73, 1996, p. 152, doi : 10.15227 / orgsyn.073.0152 ; Coll. Vol. 9, 1998, p. 88 ( PDF ).
- ↑ Kaori Ando: Highly Selective Synthesis of Z-Unsaturated Esters by Using New Horner-Emmons Reagents, Ethyl (Diarylphosphono) acetates. In: The Journal of Organic Chemistry. 62, No. 7, 1997, pp. 1934-1939, doi: 10.1021 / jo970057c .