Georg Wittig
Georg Friedrich Karl Wittig (born June 16, 1897 in Berlin ; † August 26, 1987 in Heidelberg ) was a German chemist and recipient of the 1979 Nobel Prize for Chemistry. Wittig found a way to convert the carbonyl group of an aldehyde or ketone into the carbon-carbon Convert double bond of an alkene with any substituents.
Life
Wittig was the son of a professor at the Kunstgewerbeschule in Kassel, a position later held by his younger brother, Gustav Wittig. The mother was musically gifted. Georg had the artistic talent of both parents, he played the piano very well, could also compose and paint very well. He attended Wilhelmsgymnasium in Kassel until he graduated from high school and began to study chemistry at the University of Tübingen in 1916 at the age of 19 . However, he was called up shortly afterwards and was taken prisoner by the English. From 1919 he studied chemistry in Marburg . Wittig worked there at the chemical institute under Karl Friedrich von Auwers and received his doctorate on May 7, 1923.
In the same year he got a job as a teaching assistant at the University of Marburg . He married Waltraut Ernst. After his habilitation in 1926, he worked as a senior assistant at Hans Meerwein . In 1932 he became department head and adjunct professor at the TH Braunschweig .
In 1933 Wittig became a member of the SA . In 1937 Hermann Staudinger brought him to his institute in Freiburg im Breisgau ; in the same year Wittig joined the NSDAP . In 1939 Wittig became civil servant and from 1944 he taught as full professor at the University of Tübingen . From 1956 Wittig worked as director of the organic-chemical institute of the University of Heidelberg .
He was an honorary citizen of the city of Heidelberg.
Scientific work
Wittig initially dealt with organic radicals. He made hexaphenylethane and suspected a biradical structure. However, this structure could not be confirmed. Wittig used phenyllithium to introduce phenyl groups . In 1930 Karl Ziegler found a simple method of preparing phenyllithium from bromobenzene and butyllithium. The anionic phenyllithium proved to be a very strong phenylation reagent; it could be converted to diphenyl with bromobenzene . The carbon-lithium bond is very strongly polarized. Wittig postulated dehydrobenzene on the basis of the reactions available (see picture).
Wittig later became interested in other carbon groups with an anionic charge on carbon and a positive charge. He deprotonated tetramethylammonium ions with phenyllithium and obtained amine ylides. The name ylid denotes the ionic charge in a molecule. The nitrogen normally only has three bonds to neighboring atoms. With the deprotonation via phenyllithium, a negative carbon atom was created in the vicinity of the positive nitrogen. These ylids can easily attach to polar bonds. In the presence of benzophenone, the amine ylides gave salts that were easy to characterize, the negatively charged carbon of the ylide being linked to the positively polarized carbonyl carbon. Phosphorus is very similar to nitrogen. Already August Wilhelm von Hofmann continued his successful studies of amines with phosphines continued. Wittig also went this way and was successful.
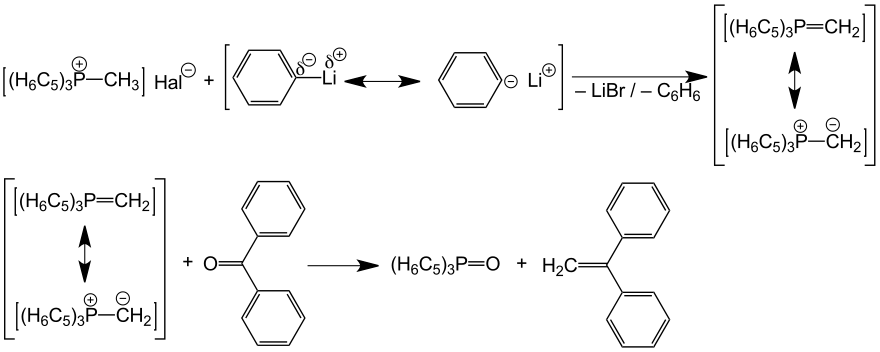
The reaction of triphenylphosphine with methyl iodide produces the quaternary phosphonium salt. With phenyllithium, this salt can be deprotonated to form triphenylphosphine methylene. In contrast to nitrogen, a resonance structure with a double bond between the methylene group and the phosphorus can be formulated with phosphorus (Ylene).
In 1954, when this ylene was reacted with benzophenone , Wittig discovered the formation of diphenylethene and the splitting off of triphenylphosphine oxide . With the deprotonated triphenylphosphine methylene, a carbonyl oxygen could be replaced by a methylene group. Instead of a simple methylene group, other ylide salts could also replace the oxygen atom of the carbonyl group with complex carbon structures. 10 years after Wittig's discovery, more than 70 patents for the conversion of substances and natural product synthesis with the Wittig reaction had already been applied for. Wittig made his great discovery at an advanced age, at the age of 57.
By 1950, he developed with his doctoral Ulrich Schöllkopf a generally applicable olefin - synthesis , named after him Wittig reaction ; for this he received the Nobel Prize in Chemistry in 1979 together with Herbert Charles Brown , who worked in the field of boranes .
The Wittig reaction is used, among other things, to produce retinol (vitamin A) on an industrial scale. On a laboratory scale, it is one of the most important reactions for the creation of C = C double bonds. Carbonyl compounds and phosphorylides are reacted with one another in an addition-elimination sequence.
The 1,2-Wittig rearrangement was named after him.
Honors
- 1953: Adolf von Baeyer commemorative coin
- 1953: Corresponding member of the Bavarian Academy of Sciences
- 1956: Full member of the Heidelberg Academy of Sciences
- 1962: Member of the German Academy of Sciences Leopoldina
- 1967: Otto Hahn Prize for Chemistry and Physics
- 1969: Corresponding member of the Académie des sciences (from 1972 external member)
- 1972: Paul Karrer Lecture
- 1979: Nobel Prize in Chemistry
- 1980: Great Cross of Merit with Star and Shoulder Ribbon of the Federal Republic of Germany
Works
- Preparative chemistry. Springer, Berlin 1976, ISBN 3-540-07932-7
- Stereochemistry. Akad. Verlagsges., Leipzig 1930.
- Via ate complexes as reaction-directing intermediates. West German Verl., Cologne 1966.
- Studies on α-oxydiphenyl and on the formation of diphenoquinones. Univ. Dissertation, Marburg 1923.
- To develop the benzo-gamma-pyrone. Habilitation thesis, Marburg 1926.
- with Ulrich Schöllkopf: About triphenyl-phosphine-methylene as olefin-forming reagents. Ber. d. German Chem. Ges. 87 (9): pp. 1318-1330, 1954.
literature
- Hans J. Bestmann: Wittig chemistry. Springer, Berlin 1983, ISBN 3-540-11907-8 .
- U. Schöllkopf: Georg Wittig. in: Chemistry in our time . 197-, p. 158ff.
Web links
- Literature by and about Georg Wittig in the catalog of the German National Library
- Information from the Nobel Foundation on the 1979 award ceremony for Georg Wittig (English)
- Wittig, Georg 1897– on worldcat.org (list of publications)
Individual evidence
- ^ Ernst Klee : The dictionary of persons on the Third Reich. Who was what before and after 1945. Fischer Taschenbuch Verlag, Second updated edition, Frankfurt am Main 2005, ISBN 978-3-596-16048-8 , p. 683.
- ↑ Sibylle Wieland (ed.): Heinrich Wieland: Natural scientist, Nobel laureate and Willstätters clock. Wiley-VCH-Verlag, Weinheim 2008, p. 91.
| personal data | |
|---|---|
| SURNAME | Wittig, Georg |
| ALTERNATIVE NAMES | Wittig, Georg Friedrich Karl |
| BRIEF DESCRIPTION | German chemist, Nobel Prize in Chemistry 1979 |
| DATE OF BIRTH | June 16, 1897 |
| PLACE OF BIRTH | Berlin |
| DATE OF DEATH | August 26, 1987 |
| Place of death | Heidelberg |