1,2-Wittig rearrangement
The 1,2-Wittig rearrangement is a name reaction in organic chemistry . It was first described in 1942 by the future Nobel Prize winner , the German chemist Georg Wittig (1897–1987) and later named after him.
In the 1,2-Wittig rearrangement, an ether is rearranged to an alcohol by means of an organolithium compound (e.g. methyllithium ) .
Reaction mechanism
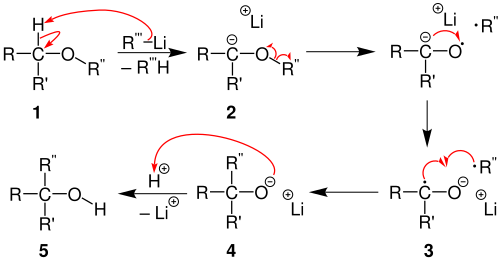
In the first step of the reaction, the ether 1 is deprotonated by means of an organolithium compound. A ketyl anion radical 3 and an alkyl radical are formed from the anion 2 through homolytic bond breaking and subsequent rearrangement . The lithium alcoholate 4 is formed through recombination of the two radicals and finally the substituted alcohol 5 through acidic processing .
Influence of the substituents
The success of the 1,2-Wittig rearrangement is largely determined by the substituents on the starting material:
At least one of the two substituents R and R ' must be able to stabilize the carbanion 2 formed in the first reaction step . Aryl , alkenyl and alkynyl radicals are therefore suitable . For the rearranged remainder R ″ , its migration tendency as a radical is decisive. There is the following order with increasing migration tendency: methyl <primary alkyl group <secondary alkyl group <tertiary alkyl group < benzyl group < allyl group .
If R ″ is an allyl group, the 1,2 rearrangement also competes with the 2,3 Wittig rearrangement .
If R or R ' is a good leaving group and an electron-withdrawing group (e.g. cyanide ), this group is split off and the corresponding ketone is formed:
Individual evidence
- ↑ Georg Wittig, Lisa Löhmann: Practice the cation-tropic isomerization of certain benzyl ethers when exposed to phenyllithium. In: Justus Liebig's Annals of Chemistry. 550, No. 1, 1942, pp. 260-268, doi : 10.1002 / jlac.19425500117 .
- ↑ G. Wittig: Results and Problems of Organic Anionochemistry. In: Experientia. 14, No. 11, 1958, pp. 389-395, doi : 10.1007 / BF02160421 .
- ↑ Jie Jack Li: Name reactions, a collection of detailed reaction mechanism . 5th edition. Springer 2014. pp. 636–637. ISBN 978-3-319-03979-4 .
- ↑ a b László Kürti , Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis . Elsevier Academic Press, Burlington / San Diego / London 2005, pp. 490-491, ISBN 0-12-369483-3 .
- ^ Sven Strunk, Manfred Schlosser: Wittig Rearrangement of Lithiated Allyl Aryl Ethers: A Mechanistic Study. In: European Journal of Organic Chemistry. 2006, No. 19, 2006, pp. 4393-4397, doi : 10.1002 / ejoc.200600304 .
- ↑ Katsuhiko Tomooka, Hiroshi Yamamoto, Takeshi Nakai: Recent Developments in the [1,2] -Wittig Rearrangement . In: Liebigs Ann. Chem. 1997, No. 7. pp. 1275-1281. doi : 10.1002 / jlac.199719970703 .
- ↑ Wolfgang Uhl, Apostolos Kyriatsouilis: Name and Keyword reactions in organic chemistry. Vieweg-Verlag 1984. pp. 160-162. ISBN 3-528-03581-1 .
- ^ Alan R. Katritzky, Yuming Zhang, Sandeep K. Singh: Preparation of aryl benzyl ketones by [1, 2] -Wittig rearrangement. In: Arkivoc. 7, 2002, pp. 146–150 ( PDF ( Memento of September 28, 2006 in the Internet Archive ); 47 kB).