Ylide
| Mesomeric boundary structures (examples) ylid (top) / ylidene (bottom) |
|
|---|---|
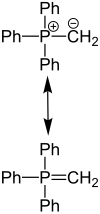 P -Ylid & P -Ylid |
 S -Ylid & S -Ylid |
An ylid is an inner salt with carbon as an anion (i.e. a zwitterion). The carbon-heteroatom bond has both covalent ("-yl") and ionic ("-id") character. The carbanionic center is stabilized by the neighboring heteroatom, there are two resonance-stabilized boundary structures, which are called ylid (zwitterion) and ylene (C = Y double bond). Ylides are C nucleophiles .
Phosphor ylides
General
Ylides often refer to phosphor ylides (also known as phosphoranes ). These are organophosphorus compounds that are usually represented in the literature in the Ylen form R 3 P = CR'R * (where R = organic radical , often phenyl ), i.e. with a double bond between the phosphorus and carbon atoms. The phosphorus atom has the oxidation state + V. Phosphorus ylides are produced by reacting phosphonium salts [R 3 P-CHR'R *] X (with X = Cl, Br, I etc.) with strong bases (for example n- butyl-lithium , n BuLi), potassium tert -butanolate (KO t Bu), sodium amide (NaNH 2 etc.>). An acidic α-H atom is necessary for the formation of ylides, which is why [Ph 4 P] X (Ph = phenyl) does not form a ylide.
use
Phosphorylides are used in the Wittig reaction . Most of the time, they are not isolated, but rather prepared in situ and reacted directly with an aldehyde , which ultimately receives the methylene group of the ylide (i.e., C = O becomes C = CH 2 ). This reaction and its variations ( e.g. Horner-Wadsworth-Emmons reaction ) are used as a key step in a large number of industrial processes (e.g. in vitamin synthesis).
Schmidbaur was the first to recognize that ylides are suitable as ligands for metals due to their donor properties (negative charge of the carbanion). He also succeeded for the first time in isolating trimethyl methylene phosphorane (Me 3 P = CH 2 ), an extremely reactive compound.
More ylids
Other ylides of main group elements are known, for example with arsenic (R 3 As = CR'R *), nitrogen (R 3 N + −C - R'R *) and sulfur (R 2 S = CR'R *) . The terms P -Ylid, N -Ylid or S -Ylid are used to distinguish the different ylids.
Since the octet rule is strictly valid for nitrogen, N -Ylides can only be formulated in an ionic boundary structure.
Individual evidence
- ↑ Reinhard Brückner : Reaction Mechanisms , Springer Verlag, 3rd edition, 2004, ISBN 978-3-8274-1579-0 , pp. 455-466.
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 497.