Sulfur ylides
| Mesomerism (example: dimethyl sulfonium methylide) ylid (top), ylidene (bottom) |
|---|

|
Sulfur ylides , also sulfonium ylides or S -ylides for short , are chemical compounds. These are internal salts with carbon as the anion and sulfur as the cation (i.e. a zwitterion ). The S -Ylides are mesomeric stabilized.
Manufacturing
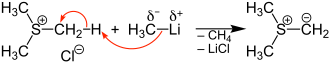
The reaction of a trialkylsulfonium halide (e.g. trimethylsulfonium chloride ) with strong bases (e.g. methyllithium ) produces an S -Ylide:
The S -Ylides are reactive intermediates and are usually immediately reacted with other substances.
Reactivity
With a ketone z. B. Dimethylsulfonium methylide to an oxirane :
Starting from an activated alkene , a cyclopropane derivative is formed analogously :
In both reactions, dimethyl sulfide (H 3 CSCH 3 ) is split off in stoichiometric amounts. In this respect, the atomic efficiency of these syntheses is low.
Individual evidence
- ↑ a b c Siegfried Hauptmann : Organische Chemie , 2nd reviewed edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 478.
- ↑ Ivan Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry , Springer-Verlag, 1972, ISBN 3-211-81060-9 , p. 200.