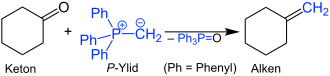Phosphor ylides
| Mesomerism (example: triphenylphosphonium methylide) ylid (top), ylidene (bottom) |
|---|

|
Phosphorus ylides , also phosphonium ylides or P -ylides for short , are chemical compounds. These are internal salts with carbon as the anion and phosphorus as the cation (i.e. a zwitterion ). The P -Ylides are mesomeric stabilized and have a strongly polar P = C double bond. In synthetic organic chemistry, P- ylides are important reagents for the production of alkenes using the Wittig reaction .
| Relative stability of triphenylphosphonium ylides |
|---|
|
unstable ylid |
|
unstable ylid |
|
semi-stable ylid |
|
stable ylid |
|
stable ylid |
Electronic structure of P -ylides
Ylid denotes a boundary structure with charge separation, the atoms involved each having an octet configuration. In the non-polar Ylen resonance structure (see above), the phosphorus atom carries ten electrons, which is why energetically high d orbitals should be involved in the bond. Structural investigations assign the highest proportion to the ylid structure, i.e. a zwitterionic with positively charged phosphorus atom and negatively charged carbon atom (carbanion). There are non-stabilized ylides in which the phosphorus atom has an alkylidene group (e.g. methylene , = CH 2 ). In the case of stabilized ylides, the negative charge on the carbon atom bound directly to the phosphorus atom is stabilized by electron-withdrawing groups such as COOR, C (= O) R, CN ...
Production of alkylidene triphenylphosphoranes
The starting material required is a phosphonium halide with at least one hydrogen atom in the α position. The reaction of this phosphonium halide (e.g. methyltriphenylphosphonium chloride ) with strong bases (e.g. methyllithium ) yields a P -ylide.
Instead of methyllithium, other strong bases such as butyllithium , phenyllithium , sodium amide or methylsulfinylcarbanions can also be used to split off the α-hydrogen atom.
Reactivity
With a ketone such as cyclohexanone , z. B. Methylentriphenylphosphoran to the corresponding alkene .
This is a simple example of a Wittig reaction . In these reactions, triphenylphosphine oxide (Ph 3 P = O) is split off in stoichiometric amounts. In this respect, the atomic efficiency of this synthesis is low. Nevertheless, vitamin A is produced on an industrial scale by a Wittig reaction
Individual evidence
- ^ A b c d Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , pp. 539-540.
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry , 2nd Edition, Springer Spectrum, 2013, ISBN 978-3-642-34715-3 , pp. 757-761.
- ↑ Reinhard Brückner: Reaction Mechanisms , Springer Verlag, 3rd edition, 2004, ISBN 978-3-8274-1579-0 , pp. 455-466.
- ↑ Ivan Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry , Springer-Verlag, 1972, ISBN 3-211-81060-9 , p. 200.
- ↑ Werner Reif, Hans Grassner: The technical vitamin A synthesis of BASF . In: Chemical Engineer Technology . tape 45 , no. 10 , 1973, p. 646-652b , doi : 10.1002 / cite.330450920 .