Cyclohexanone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cyclohexanone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 10 O | ||||||||||||||||||
| Brief description |
colorless liquid with a peppermint odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 98.15 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.95 g cm −3 (20 ° C ) |
||||||||||||||||||
| Melting point |
−26 ° C |
||||||||||||||||||
| boiling point |
156 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.4507 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
In its pure state, cyclohexanone is a colorless, water-clear liquid with a smell reminiscent of acetone . It belongs to the group of cyclic ketones ( cycloalkanones ).
Extraction and presentation
There are two main processes available for the technical synthesis of cyclohexanone:
1) The catalytic oxidation of cyclohexane with atmospheric oxygen, which takes place via the unstable cyclohexyl hydroperoxide and follows a radical mechanism . A mixture of cyclohexanone and cyclohexanol is formed, which is also known as anon and anol for short . The mixture can be separated by distillation .
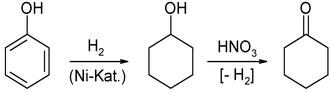
2) The oxidation of cyclohexanol with dilute nitric acid or in the gas phase over zinc oxide catalysts. The cyclohexanol previously by hydrogenation of phenol produced over a nickel catalyst:
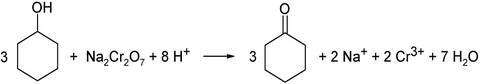
On a laboratory scale , cyclohexanone can also be produced by oxidation of cyclohexanol in acidic solution with chromium (VI) salts , e.g. B. sodium dichromate can be obtained. This reaction takes place mechanistically via a chromic acid ester of cyclohexanol:
The enzymatic double oxidation of cyclohexane to cyclohexanone is also described in the literature.
properties
Physical Properties
Cyclohexanone is a colorless liquid at room temperature with a density of 0.947 g / cm³. It solidifies at −31 ° C and boils at 155 ° C under normal pressure . It is miscible with most common organic solvents in any ratio. The miscibility with water is limited. The solubilities at 20 ° C. are 9.0 g of cyclohexanone in 100 g of water and 5.7 g of water in 100 g of cyclohexanone. These solubilities are temperature dependent. As the temperature rises, the solubility of cyclohexanone in water decreases or the solubility of water in cyclohexanone increases. With water there is a constant boiling ( azeotropic ) mixture at 96 ° C and 57% water.
Solubilities between cyclohexanone and water temperature ° C 0 9.8 19.5 29.8 40.1 50.2 60.5 71.1 80.2 90.7 Cyclohexanone in water in% 13.7 11.5 9.7 8.2 7.5 7.0 6.7 6.5 6.8 6.9 Water in cyclohexanone in% 4.69 5.05 5.35 5.93 6.28 6.81 7.34 8.15 8.84 9.82
Chemical properties
Cyclohexanone shows the typical reactions for ketones, for example:
- Imine formation with ammonia and primary amines ,
- Enamine formation with secondary amines (see below ),
- CH acidity of the two CH 2 groups, which are adjacent to the carbonyl group , and the associated enolizability ,
- Aldol reactions with base or acid catalysis,
- Reaction with Grignard reagents,
- Olefin formation under the conditions of the Wittig reaction or the Horner-Wadsworth-Emmons reaction ,
Etc.
It can be easily reduced to cyclohexanol with sodium borohydride .
Of concentrated (65%) nitric acid is at 30-40 ° C under ring opening to give adipic acid oxidized. For the synthesis of adipic acid, however, one starts from cyclohexanol, the cyclohexanone initially formed after the reaction listed above reacting immediately.
Safety-related parameters
Cyclohexanone forms flammable vapor-air mixtures. The compound has a flash point of 43 ° C. The explosion range is between 1.3% by volume (53 g / m³) as the lower explosion limit (LEL) and 9.4% by volume (380 g / m³) as the upper explosion limit (UEL). The lower explosion point is 38 ° C. The limit gap width was determined to be 0.95 mm. This results in an assignment to explosion group IIA. The ignition temperature is 430 ° C. The substance therefore falls into temperature class T2.
use
Probably the most important industrial use of cyclohexanone is its conversion to ε-caprolactam, which is used to manufacture Perlon . First, through condensation with hydroxylamine (used in the form of hydroxylamine hydrochloride ), the cyclohexanone oxime is formed, which is converted to ε-caprolactam in a Beckmann rearrangement :
It is a typical representative of the cyclic ketones, especially in practical experiments in chemical training. For example, the formation of enamines or the α-alkylation with cyclohexanone as the starting material work very well:
Due to its diverse reaction possibilities as a ketone, it is often used in synthetic organic chemistry when six-membered structural elements are to be incorporated into molecular structures.
Cyclohexanone is also a very good solvent for paint raw materials, polyvinyl chloride , basic dyes as well as natural and synthetic resins . The material is also contained in opaque paints for leather , printing inks and paint strippers .
When determining zinc with zincon, cyclohexanone is used as a selective unmasking reagent.
safety instructions
Cyclohexanone is particularly harmful if inhaled and can cause dizziness and headaches. It also forms flammable vapors when heated. Therefore, it should be handled under a well-pulling hood . Protective gloves and goggles are to be worn.
Risk assessment
Cyclohexanone was included in the EU's ongoing action plan ( CoRAP ) in 2016 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of cyclohexanone were concerns about worker exposure , high (aggregated) tonnage and widespread use as well as the possible dangers of carcinogenic, mutagenic and reproductive toxicity properties. The re-evaluation took place from 2016 and was carried out by Poland . A final report was then published.
literature
- Beyer / Walter: Textbook of Organic Chemistry. Hirzel-Verlag Stuttgart, 23rd edition 1998, pp. 306, 353 and 430
Web links
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r s Entry on cyclohexanone in the GESTIS substance database of the IFA , accessed on May 5, 2018(JavaScript required) .
- ↑ Entry on cyclohexanone. In: Römpp Online . Georg Thieme Verlag, accessed on December 22, 2014.
- ↑ Cyclohexanone data sheet at AlfaAesar, accessed on May 18, 2016 ( PDF )(JavaScript required) .
- ↑ Entry on cyclohexanone in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 108-94-1 or cyclohexanone ), accessed on November 2, 2015.
- ↑ Svenja Staudt, Edyta Burda, Carolin Giese, Christina A. Mller, Jan Marienhagen, Ulrich Schwaneberg, Werner Hummel, Karlheinz Drauz and Harald Gröger: Direct oxidation of cycloalkanes to cycloalkanones with oxygen in water, Angewandte Chemie 125 (2013) p. 2415– 2419.
- ↑ MT Musser: Cyclohexanol and Cyclohexanone in Ullmann's Encyclopedia of Industrial Chemistry, 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, doi : 10.1002 / 14356007.a08_217.pub2 .
- ↑ a b R.M. Stephenson: Mutual Solubilities: Water-Ketones, Water-Ethers, and Water-Gasoline-Alcohols in J. Chem. Eng. Data 37 (1992) 80-95, doi: 10.1021 / je00005a024 .
- ^ IM Smallwood: Handbook of organic solvent properties , Arnold London 1996, ISBN 0-340-64578-4 , pp. 183-185.
- ^ A b c E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Cyclohexanone , accessed on March 26, 2019.