Beckmann rearrangement
The Beckmann rearrangement is a name reaction from the field of organic chemistry and was named after its discoverer, the German chemist Ernst Otto Beckmann (1853–1923). The rearrangement is used for the synthesis of carboxamides .
Overview reaction
In this reaction, an acid-catalyzed conversion of ketoximes or aldoximes into carboxamides takes place . With further hydrolysis , the Beckmann rearrangement in aldoximes ultimately leads to the amide .
If it is a ketoxime, R 1 and R 2 are organyl residues . In the case of aldoximes, one of the residual groups is a hydrogen atom.
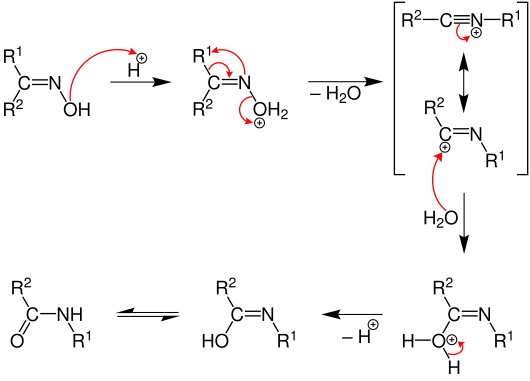
Reaction mechanism
The Beckmann rearrangement begins with the protonation of the ketoxime on the hydroxyl group . The water is split off and one of the two residues migrates to nitrogen. The group that is trans to the hydroxyl group always migrates . After nucleophilic attack by water and deprotonation, the iminol form is formed, which then tautomerizes to the acid amide . Since the oxime group is angled on the nitrogen, different radicals R 1 and R 2 have ( E / Z ) isomers .
The representation of nitriles by "dehydration" of aldoximes with z. B. Phosphorus pentachloride , PCl 5 , is based on an analogous mechanism:
application
The Beckmann rearrangement is important in the technical production of ε- caprolactam , the starting material for Perlon production. Here, cyclohexanone oxime is rearranged in the presence of sulfuric acid:
literature
- L. Guy Donaruma, WM Heldt: The Beckmann rearrangement. In: Organic Reactions. 11, 1960, pp. 1-156.
- Robert E. Gawley: The Beckmann Reactions: Rearrangements, Elimination-Additions, Fragmentations, and Rearrangement-Cyclizations. In: Organic Reactions. 35, No. 1, 1988, pp. 14-24 doi : 10.1002 / 0471264180.or035.01 .
Individual evidence
- ↑ Ernst Beckmann: On the knowledge of the isonitroso compounds. In: Reports of the German Chemical Society. 19, No. 1, 1886, pp. 988-993, doi : 10.1002 / cber.188601901222 .
- ↑ Peter Sykes: Reaction Mechanisms - An Introduction. 8th edition VCH, Weinheim 1982, ISBN 3-527-21090-3 , p. 141.
- ↑ Eck, JC; Marvel, CS: ε-Benzoylaminocaproic Acid In: Organic Syntheses . 19, 1939, p. 20, doi : 10.15227 / orgsyn.019.0020 ; Coll. Vol. 2, 1943, p. 76 ( PDF ).