Hydroxylamine hydrochloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
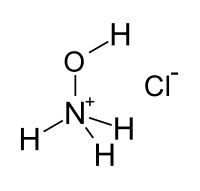
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hydroxylamine hydrochloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | [NH 3 OH] Cl | |||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 69.49 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.67 g cm −3 |
|||||||||||||||
| Melting point |
159 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Hydroxylamine hydrochloride , also known as hydroxylammonium chloride , is a colorless, crystalline inorganic chemical compound . It is the hydrochloric acid salt of hydroxylamine and behaves like unbound hydroxylamine in most chemical reactions , but is more thermally stable .
Manufacturing
Hydroxylamine hydrochloride is accessible through
- Electrolysis of a mixture of mercury nitrate , sulfuric acid and nitric acid (this creates the sulfate) and subsequent addition of barium chloride solution .
- by boiling potassium hydroxylamine disulfonate , forming the sulfate as above, from which the product with barium chloride is obtained.
properties
Hydroxylamine hydrochloride reacts with aldehydes or ketones to form oximes and with carboxylic acids to form hydroxamic acids .
use
Hydroxylamine hydrochloride is a versatile reagent in pharmaceutical and organic synthesis, for example for the synthesis of oximes and oxime ethers from carbonyl compounds or for the synthesis of hydroxamic acids. In analysis it is used in oxime titration .
Legal status
In Germany, hydroxylamine hydrochloride is classified as an explosive substance of substance group C in accordance with the regulations of the Explosives Act.
literature
- Entry on hydroxylammonium chloride. In: Römpp Online . Georg Thieme Verlag, accessed on July 15, 2014.
Individual evidence
- ↑ a b entry on hydroxylammonium chloride. In: Römpp Online . Georg Thieme Verlag, accessed on July 15, 2014.
- ↑ a b c d e Entry on hydroxylammonium chloride in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Entry on Hydroxylammonium chloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ G. Brauer (Ed.), Handbook of Preparative Inorganic Chemistry 2nd ed., Vol. 1, Academic Press 1963, pp. 487-8.
- ↑ Announcement of the new findings made by BAM since 1987 in accordance with Section 2 SprengG . (PDF)




