Oxime ethers
Oxime ethers (spoken: oxime ethers) are organic chemical compounds that are derived from the oximes with the functional group C = N − OH in that the hydrogen atom of the hydroxyl group is substituted by an alkyl radical .
Manufacturing
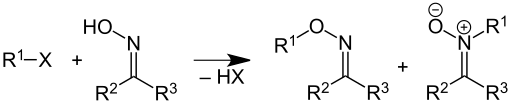
Oxime ethers are synthesized from oximes ( aldoximes or ketoximes ) and haloalkanes or alkyl sulfates . By-products are formed by N -alkylation nitrones . The relative yield of oxime ether and nitrone formed depends on the reaction conditions and reagents. Thus forming anti -Benzaloxim (oxime of benzaldehyde ) mainly nitrones, while the syn - isomer mainly responding to oxime ether.
When silver salts of oximes are reacted with alkyl iodides in ether or alcohol, oxime ethers are obtained almost exclusively, nitrones are not formed. When aldoximes are reacted with diazomethane as the methylating agent, mixtures of O- methyl oximes and methyl nitrones are obtained. The oxirane ring O- (2-hydroxyalkyl) -oximes is obtained from oximes and oxiranes with opening .
Individual evidence
- ↑ Jerry March : Advanced Organic Chemistry , Wiley, 3rd Edition (1985), p. 359, ISBN 0-471-85472-7 .
- ↑ Buchler, J. Org. Chem. 32 ( 1967 ) 261.
- ↑ Horst Metzger: Production and conversion of oximes in Houben-Weyl (editors: Eugen Müller, Otto Bayer, Hans Meerwein and Karl Ziegler): Methods of Organic Chemistry, Volume X / 4, Nitrogen Compounds I, Part 4, 1968, p. 1 -308, there pp. 219-220.
- ↑ Horst Metzger: Production and conversion of oximes in Houben-Weyl (editors: Eugen Müller, Otto Bayer, Hans Meerwein and Karl Ziegler): Methods of Organic Chemistry, Volume X / 4, Nitrogen Compounds I, Part 4, 1968, p. 1 –308, there p. 223.
- ↑ Horst Metzger: Production and conversion of oximes in Houben-Weyl (editors: Eugen Müller, Otto Bayer, Hans Meerwein and Karl Ziegler): Methods of Organic Chemistry, Volume X / 4, Nitrogen Compounds I, Part 4, 1968, p. 1 –308, there p. 224.