Benzaldehyde
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
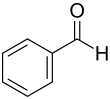
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Benzaldehyde | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 6 O | |||||||||||||||
| Brief description |
colorless to yellowish liquid with a bitter almond odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 106.13 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.05 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−26 ° C |
|||||||||||||||
| boiling point |
179 ° C |
|||||||||||||||
| Vapor pressure |
1.26 hPa (20 ° C) |
|||||||||||||||
| solubility |
heavy in water (6.95 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.5446 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Benzaldehyde [ ˈbɛnt͡s | aldehyːt ] (also called "artificial bitter almond oil ") is a colorless to yellowish liquid with a bitter almond odor. Benzaldehyde is the simplest aromatic aldehyde . It is derived from benzene and is chemically and structurally closely related to benzoic acid , which can be obtained from it by oxidation . It is an important basic chemical .
presentation
Free radical chlorination of toluene gives benzal chloride (dichloromethylbenzene), which can be reacted with water to form benzaldehyde (and HCl ). Accordingly, benzaldehyde is also obtained by radical bromination of toluene and subsequent hydrolysis . In both cases, a nucleophilic substitution of halide occurs in the last reaction step .
A 'direct' oxidation of toluene to benzaldehyde is also possible. However, since benzaldehyde is easily oxidized to benzoic acid, either selective oxidizing agents must be used or the benzaldehyde formed must be 'captured' with a rapid reaction and thus protected from further oxidation.
properties
Benzaldehyde is harmful to health, although a real health hazard is usually only to be expected if relatively large amounts are ingested. It smells pleasantly sweet like marzipan. The taste of benzaldehyde is generally perceived as characteristic marzipan-like , but in its pure state it is also felt to be unpleasantly burning. In great dilution, especially with ethanol , a wild cherry note is increasingly added to the aroma . Benzaldehyde is also one of the flavors in wine . With an odor threshold of 3 milligrams / l in white wine , it is an important component of the wine bouquet . Benzaldehyde has a flash point of 64 ° C and an ignition temperature of 190 ° C. The aroma of marzipan results for the most part from the aromas of the almonds used or their roast aromas. Benzaldehyde is an important component of the aroma.
Reactions
- Benzaldehyde C 6 H 5 -CHO is easily oxidized to benzoic acid C 6 H 5 -COOH. This reaction takes place - albeit very slowly - at room temperature and with atmospheric oxygen ( autoxidation ), so that benzaldehyde is often contaminated with benzoic acid (in larger quantities as a white solid in the liquid aldehyde). Peroxybenzoic acid is formed as an intermediate stage .
- Benzaldehyde enters into typical reactions for aldehydes , which are also suitable for detection . It reacts, for example, with hydrazine H 2 N-NH 2 and its derivatives (for example with phenylhydrazine C 6 H 5 -NH-NH 2 to form a phenylhydrazone ).
- The electrophilic substitution typical of aromatics is also possible with benzaldehyde. During the nitration , the reaction conditions must be chosen carefully, since the oxidation to benzoic acid occurs as a side reaction . For this reason, the yield of nitrobenzaldehyde is usually below 50%.
- Benzoin addition : Two molecules of benzaldehyde can combine to form benzoin in the presence of cyanide as a catalyst . In general, benzoin addition is understood to mean the corresponding reaction of aromatic aldehydes (with benzaldehyde as the simplest representative).
- Aldol condensation : This is generally understood to mean the addition of carbonyls to aldehydes . For example, dibenzalacetone can be represented in this way. A ketone ( acetone ) initially reacts in the basic form to a so-called methylene component. After adding benzaldehyde (carbonyl component ), this reacts to form benzalacetone (benzylidene acetone). If a further addition is carried out using benzaldehyde, the benzalacetone reacts this time to form the methylene component in a basic medium. The second addition or double aldol condensation can be used to synthesize complex molecules such as dibenzalacetone (1,5-diphenylpenta-1,4-dien-3-one).
- During hydrate formation, water is added to the aldehyde group of the carbonyl compound with nucleophilic attack. A disadvantage of this reaction is the instability of hydrates in general.
- In the reaction with primary amines (R – NH 2 ), an azomethine is also formed with nucleophilic attack on the central C atom of the carbonyl group and subsequent elimination of water ( condensation ) from the intermediate product .
safety instructions
Benzaldehyde was included in the EU's ongoing action plan ( CoRAP ) in 2016 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. Benzaldehyde uptake was caused by concerns about consumer use , worker exposure and widespread use, as well as the potential risk of mutagenic properties. The re-evaluation is to be carried out by France from 2020 .
Individual evidence
- ↑ a b c d e f g h i j Entry on benzaldehyde in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ^ Association of authors: Organikum . 22nd edition. Wiley-VCH, 2004, ISBN 3-527-31148-3 , p. 236.
- ↑ Entry on benzaldehyde in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Rainer Amann: How does the black currant actually get into the Scheurebe? (PDF; 2.1 MB) In: The Badische Winzer. October 2002.
- ^ Mitchell, Alyson E .: Beyond benzaldehyde: The chemistry of raw, roasted and rancid almonds . In: Abstracts of Papers, 253rd ACS National Meeting & Exposition, San Francisco, CA, United States, April 2-6, 2017 . tape 253 , 2017, p. AGFD-170 .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Benzaldehyde , accessed on March 26, 2019.
