4-phenyl-3-buten-2-one
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
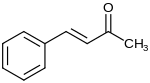
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 4-phenyl-3-buten-2-one | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 10 O | ||||||||||||||||||
| Brief description |
light yellow solid with a characteristic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 146.19 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.008 g cm −3 |
||||||||||||||||||
| Melting point |
38-41 ° C |
||||||||||||||||||
| boiling point |
261 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
4-Phenyl-3-buten-2-one (benzylidene acetone) is a chemical compound from the group of ketones .
Occurrence
4-Phenyl-3-buten-2-one occurs naturally in soybeans and Virginia tobacco .
Extraction and presentation
4-Phenyl-3-buten-2-one can be obtained by reacting acetone with benzaldehyde in the presence of sodium hydroxide ( Claisen-Schmidt condensation ).
properties
4-Phenyl-3-buten-2-one is a flammable, hardly ignitable, light yellow solid with a characteristic odor (smell of coumarin ), which is sparingly soluble in water. trans -4-phenyl-3-buten-2-one is a substrate for glutathione-S-transferases . It reacts with methyl and benzylguanidines to form aromatic N 2 -substituted 2-pyrimidinamines.
use
Due to its enone substructure, 4-phenyl-3-buten-2-one is used in organic synthesis as a building block for condensation , cycloaddition , Michael additions and Grignard reactions . With diiron nonacarbonyl [Fe 2 (CO) 9 ] it provides benzylidene iron tricarbonyl, a reagent that can transfer the Fe (CO) 3 group to other molecules . Benzalacetone is used as a starting material for the synthesis of warfarin .
safety instructions
4-Phenyl-3-buten-2-one must not be used in the manufacture or treatment of cosmetic products (German Cosmetics Ordinance Annex 1, No. 356).
Individual evidence
- ↑ a b c d e f g h i j Entry on 4-phenyl-3-buten-2-one in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b Data sheet 4-Phenyl-3-buten-2-one, 99% from Sigma-Aldrich , accessed on January 18, 2016 ( PDF ).
- ↑ George A. Burdock: Fenaroli's Handbook of Flavor Ingredients, Sixth Edition . CRC Press, 2009, ISBN 978-1-4200-9086-4 ( limited preview in Google Book Search).
- ↑ Drake, NL; Allen, Jr. P .: Benzalacetone In: Organic Syntheses . 3, 1923, p. 17, doi : 10.15227 / orgsyn.003.0017 ( PDF ).
- ↑ a b c Entry on benzylidene acetone. In: Römpp Online . Georg Thieme Verlag, accessed on January 18, 2016.
- ↑ Enders, E .: Rodenticides . In: Wegler, R. (Ed.): Chemistry of pesticides and pesticides. 1st edition. tape 1 . Springer, Berlin / Heidelberg / New York 1970, pp. 618 .

