Grignard reaction
The Grignard reaction [ ɡriˈɲaːr- ] is a name reaction in organic chemistry , which was named after its discoverer, the French chemist Victor Grignard, who was awarded the Nobel Prize in 1912 for this discovery . The Grignard reaction is an organometallic chemical reaction in which alkyl or aryl magnesium halides (see Grignard compound ) act as a nucleophile with electrophilic groups such as e.g. B. carbonyl groups react. It is used to build carbon-carbon single bonds.
The Grignard reaction is a very important reaction for making carbon-carbon, carbon- phosphorus , carbon- tin , carbon- silicon , or carbon- boron bonds. The magnesium-organic Grignard compound is not an ionic compound. Instead, depending on the solvent, there are different structures that are in equilibrium with one another, see Schlenk equilibrium .
Since Grignard compounds are sensitive to protic solvents, the Grignard reaction must be carried out anhydrous, usually in dried ethers such as diethyl ether or tetrahydrofuran .
Overview reaction
In the Grignard reaction, a Grignard compound (organometallic compound, here with bromine as an example of a halogen ) reacts as a nucleophile with an electrophilic compound - here e.g. B. the carbonyl group in an aldehyde or ketone - to an alcohol:
mechanism
Mechanistically speaking, the Grignard reaction is a nucleophilic addition in which the negatively polarized carbon atom ( carbanion ) of the Grignard compound is added to the carbon atom of a carbonyl group . A new carbon-carbon bond is thus formed. It is believed that two molecules of the Grignard compound are involved in the transition state of the reaction , resulting in a six-membered transition state. The oxygen atom of the carbonyl compound takes over the metal to form a metal alkoxide . In the next step, this oxygen atom is protonated by dilute aqueous acid .
Implementations
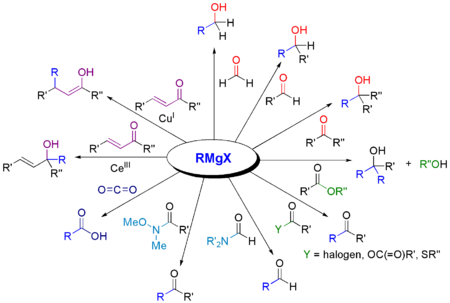
In the following, the general formula RMgX is often used for the Grignard compound, where the X stands for one of the halogens bromine , chlorine or iodine . The Grignard reaction delivers primary , secondary or tertiary alcohols , depending on the carbonyl compound used .
With the addition of two equivalent Grignard compounds, esters produce tertiary alcohols , with a single addition initially forming a ketone as an intermediate . However, this cannot be isolated because it reacts faster with the Grignard compound than the ester used.
In addition to aldehydes , ketones and esters, carbon dioxide , nitriles , imines , epoxides , thioesters etc. can also be reacted with Grignard compounds:
The most important application, however, is the reaction of a Grignard compound with aldehydes, ketones or esters to produce alcohols.
| Educt | product |
|---|---|
| formaldehyde | primary alcohol |
| aldehyde | secondary alcohol |
| Ketone | tertiary alcohol |
| Formic acid ester | secondary alcohol |
| Carboxylic acid ester | tertiary alcohol |
| Carbon dioxide | Carboxylic acid |
| Nitrile | Ketone |
| Orthoformic acid ester ( Bodroux-Tschitschibabin aldehyde synthesis ) | aldehyde |
| N, N-dialkylated formamides ( Bouveault aldehyde synthesis ) | aldehyde |
| Carbon disulfide | Dithiocarboxylic acid |
A Grignard reaction can be carried out with a number of other carbon electrophiles.
The Grignard reaction can also be used to produce various other element-carbon bonds. Particularly noteworthy here are boron compounds, which are required, for example, for the Suzuki coupling .
In the past, the Grignard reaction was of great industrial importance in the production of the fuel additive tetraethyl lead , which is now irrelevant due to the ban.
Aryl-alkyl couplings
With the aid of the Grignard reaction, aryl-alkyl couplings can be carried out in the presence of iron (III) acetylacetonate .
Also nickel chloride ( Kumada coupling ) or dilithium tetrachlorocuprate ( in situ prepared from lithium chloride and copper (II) chloride in tetrahydrofuran) are good catalysts for such coupling reactions.
Stereoselective Grignard reaction
Since many ketones and all aldehydes (except formaldehyde ) are prochiral compounds, a Grignard reaction very often results in enantiomer pairs or, if a stereocenter is already present, diastereomers . To extend the Grignard reaction stereoselectively, Dieter Seebach developed the chiral reagent TADDOL , a tartaric acid derivative. Using the Schlenk equilibrium , enantiomeric excesses of 84–96% were achieved in the reaction with aldehydes .
Competitive reactions
In the case of sterically complex Grignard compounds or sterically hindered carbonyl compounds, side reactions can take place. The most important ones are the reduction of the carbonyl component, referred to as Grignard reduction , and, if an α- hydrogen atom is present, the enolization of the carbonyl compound.
Practical implementation
In the laboratory, the Grignard compounds required are usually produced in situ in an ether as a solvent and reacted immediately with the other component. The reaction mixture is then worked up in a slightly or strongly acidic aqueous solution and the reaction product is isolated therefrom.
use
One application of the Grignard reaction for the quantitative determination of CH-acidic compounds is the Zerewitinow reaction .
See also
Web links
Individual evidence
- ^ Nobelprize.org on the award of the prize to V. Grignard .
- ^ V. Grignard: Sur quelques nouvelles combinaisons organométalliques du magnèsium et leur application à des synthèses d'alcools et d'hydrocarbures . In: CR Hebd. Séances Acad. Sci., Ser. C . tape 130 , 1900, pp. 1322–1324 ( digitized version in Gallica - French; German about some new organometallic compounds of magnesium and their application to the synthesis of alcohols and hydrocarbons ).
- ^ DA Shirley: In: Organic Reactions. Vol. 8, 1954, pp. 28-58.
- ↑ DM Huryn: In Comp. Org. Syn. 1, 1991, pp. 49-75.
- ^ C. Elschenbroich, A. Salzer: Organometallics - A Concise Introduction. 2nd Ed., Wiley-VCH, Weinheim 1995, ISBN 3-527-28164-9 , pp. 43-44.
- ↑ Kazuhiro Maruyama, Toshimasa Katagiri: Mechanism of the Grignard reaction . In: Journal of Physical Organic Chemistry . tape 2 , no. 3 , 1989, pp. 205-213 , doi : 10.1002 / poc.610020303 .
- ↑ KPC Vollhardt, NE Schore: Organic Chemistry . Ed .: H. Buntenschön. 4th edition. Wiley-VCH, Weinheim 2005, ISBN 978-3-527-31380-8 , pp. 351 .
- ↑ Jerry March: Advanced Organic Chemistry. John Wiley & Sons, New York 1985, ISBN 0-471-88841-9 .
- ^ A. Suzuki, PJ Stang (Ed.), F. Diedrich (Ed.): Metal-Catalyzed Cross-coupling Reactions. Wiley-VCH, Weinheim 1998.
- ^ C. Elschenbroich, A. Salzer: Organometallics - A Concise Introduction. 2nd Ed., Wiley-VCH, Weinheim 1995, ISBN 3-527-28164-9 , p. 139.
- ↑ A. Fürstner, A. Leitner, G. Seidel: 4-Nonylbenzoic Acid In: Organic Syntheses . 81, 2005, pp. 33-42, doi : 10.15227 / orgsyn.081.0033 ( PDF ).
- ↑ Joanna Linda von dem Bussche-Hünnefeld, Dieter Seebach: Enantioselective preparation of sec . Alcohols from aldehydes and dialkyl zinc compounds, generated in situ from Grignard reagents, using substoichiometric amounts of TADDOL-titanates . In: Tetrahedron . tape 48 , no. 27 , July 3, 1992, pp. 5719-5730 , doi : 10.1016 / 0040-4020 (92) 80023-9 .
- ↑ Reinhard Brückner: reaction mechanisms . 3rd about. and actual Edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 429f.