Ester
| Ester |
|---|
 Carboxylic acid ester |
 Mono ester of phosphoric acid |
 Mono ester of sulfuric acid |
 Nitric acid ester |
 Ester of nitrous acid |
 Boric acid tri ester |
In chemistry, esters are a group of chemical compounds that form formally or de facto through the reaction of an acid and an alcohol or phenol with the elimination of water (a condensation reaction ). There are esters of organic acids (e.g. carboxylic acids such as acetic acid , sulfonic acids ) and those of inorganic acids (e.g. phosphoric acid , sulfuric acid , boric acid , carbonic acid ).
The term ester was formed by the chemist Leopold Gmelin in 1850 from the term “vinegar ether”, a historical name for ethyl acetate . The vapors of ethyl acetate have an anesthetic effect, similar to those of "ether" ( diethyl ether ), hence the term "vinegar ether".
The production of esters is known as esterification or ester formation.
Carboxylic acid ester
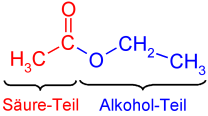
Carboxylic acid esters are esters of carboxylic acids with the functional group –COOR. They are composed of an acid part and an alcohol part .
Carboxylic acid esters form a group of substances that are often found in organic chemistry and nature ( fruit esters , fats , oils).
Biologically important esters are the triglycerides (also known as “glycerol triesters”, more rarely out of date “neutral fats”). These natural oils (liquid state of aggregation) or fats (solid) are almost all poorly soluble in water, although they are polar, because they have (with a few exceptions) three hydrophobic alkyl groups . The longer the alkyl groups, the poorer the solubility of the triester in water.
Phosphoric acid ester
Phosphoric acid esters are esters of orthophosphoric acid , which formally or actually arise from the reaction of the acid with an alcohol with elimination of water. Nucleic acids are (as part of their structure) esters of phosphoric acid with the alcohol function of sugars (e.g. ribose or deoxyribose ). In terms of structure, a distinction is made between monoester, diester and triester of ortho-phosphoric acid. Among the esters of phosphoric acid and its derivatives , compounds are known to be potent insecticides (e.g. E605 ). The derivatives also include highly toxic compounds, such as the chemical warfare agents sarin , tabun and soman .
Sulfuric acid ester
Sulfuric acid estersor alkyl sulfates are esters of sulfuric acid . They are widely represented in nature. (Examples: carrageenan , heparin or the so-called sulfatides as components of the brain matter). Salts of long-chain monoalkylsulfuric acid esters, often referred to as fatty alcohol sulfates , are used in cosmetic products as anionic surfactants (example: sodium lauryl sulfate ).
Dimethyl sulfate and diethyl sulfate are dialkyl esters of sulfuric acid and are used as powerful reagents in chemistry to transfer methyl or ethyl groups to other molecules. These dialkyl esters of sulfuric acid are toxic and carcinogenic because of their alkylating effect .
Nitric acid ester
Nitric acid esters are esters of nitric acid. The nitro group (NO 2 ) it contains is mesomeric stabilized .
Special esters
There are esters of acids that are unstable as free acids and can only exist as derivatives (e.g. orthocarbonic acid esters or carbamic acid esters ). On the other hand, it also happens that the alcohol component of an ester does not exist as a free compound, since it would rearrange and is only stabilized by the ester compound with an acid (e.g. vinyl acetate ).
Examples of esters of some other acids
- Sulfonic acid esters, the so-called sulfonates
- Nitroglycerin ( explosives and drugs , chemically correct name is glycerol trinitrate)
- Esters of nitrous acid can be produced using the Chrétien-Longi reaction .
- Boric acid trimethyl ester (detection of boron by green flame color)
- Silicic acid ester
- Carbonic acid diethyl ester
- Lactic acid ester
- Triphosgene (synthon for phosgene)
- Chromic acid ester
Use and occurrence
Only exemplary cases are given below, since the ester group occurs in a large number of molecules.
- Fats and fatty oils (esters of glycerine and fatty acids , e.g. triglycerides ) are important food components and energy storage substances for most animal organisms.
- Monoglycerides (esters from equimolar amounts of glycerine and a fatty acid) are emulsifiers in food production,
- Biodiesel is a methyl ester of fatty acids .
- Beeswax consists mainly of esters of myricyl alcohol (C 30 H 61 OH), e.g. B. Myricyl palmitate (C 15 H 31 -COO-C 30 H 61 ).
- Numerous esters are used as flavorings , some are therefore also referred to as fruit esters . This is how it smells. B. Acetic acid 2-butyl ester [CH 3 –COO – CH (CH 3 ) C 2 H 5 ] according to apple, ethanoic acid 2-methyl-1-propyl ester [CH 3 –COO – CH 2 CH (CH 3 ) 2 ] (Common name isobutyl acetate ) according to banana, 2-hexyl ethanoate [CH 3 - COO - CH (CH 3 ) (C 4 H 9 )] according to strawberry.
- The cellulose ester cellulose nitrate is used to produce smokeless propellant powder ( gunpowder ) and the plastic celluloid , which is still used today for the manufacture of table tennis balls .
- Polyester are used as plastics, both as a material such as PET ( P oly E Thylen T erephthalat), which is used for producing food packaging, and beverage bottles, as also find application in textiles.
- Esters are also used in plasticizers . Examples of this would be esters of the alkyl sulfonic acid Mesamoll and the phthalic acid ester diethylhexyl phthalate ( DEHP ) as plasticizers for PVC .
- Medicinal substances are often esterified to make them more lipophilic so that they can more easily pass through barriers such as the blood-brain barrier . By metabolism resulting from this prodrug the active form then. One example is heroin , which, through double acetylation , reaches the central nervous system and thus reaches the opioid receptors better than the active form morphine . Also acidic drugs such. B. ACE inhibitors are often esterified. Also salicylic acid is to acetylsalicylic acid is esterified (for the treatment of pain, inflammation and fever) to take on the stomach to the substance. Amyl nitrite ((CH 3 ) 2 CH – CH 2 –CH 2 –O – N = O) or glycerol trinitrate , like other alkyl nitrites and nitrates, have a relaxing effect on the smooth vascular muscles by releasing nitric oxide and are sometimes used in treatment to this day Angina pectoris attacks or used in intensive care medicine to lower blood pressure .
- Esters are used as insecticides and chemical weapons , e.g. B. phosphoric acid esters . The former include parathion and dichlorvos , the latter include sarin (isopropyl methylfluorophosphonate), soman (1,2,2-trimethylpropyl methylfluorophosphonate) and tabun (ethyl dimethylphosphoramidocyanidate).
- Sulfuric acid esters, more precisely fatty alcohol sulfates , such as. B. sodium lauryl sulfate , are used as surfactants in cosmetics.
- Glycerin trinitrate (also nitroglycerin, O 2 NO-CH 2 -CH (ONO 2 ) -CH 2 -ONO 2 ) is used as an explosive and as a drug.
- Phorbol esters are contained in the juice of milkweed plants and, in addition to severe skin burns and gastroenteritis, can also trigger malignant tumors . The carcinogenic , i.e. cancer-promoting effect seems to come about through stimulation of protein kinase C , which modifies the growth behavior of cancer cells.
Carboxylic acid ester - Occurrence in fruits (selection) as a component that gives off smell and taste
Web links
Individual evidence
- ↑ Wolfgang Legrum: Fragrances, between stink and fragrance , Vieweg + Teubner Verlag (2011) pp. 85–86, ISBN 978-3-8348-1245-2 .
- ^ MD Lechner, K. Gehrke and EH Nordmeier: Makromolekulare Chemie . 4th edition. Birkhäuser, Basel / Boston / Berlin 2010, ISBN 978-3-7643-8890-4 , pp. 126-130.
- ↑ Fritz Röthemeyer, Franz Sommer: Rubber technology . 2nd Edition. Hanser, Munich / Vienna 2006, ISBN 978-3-446-40480-9 , pp. 335–337.












