Carbamates
| Carbamates |
| Carbamate ester - ester of carbamic acid - with the carbamate group marked in blue . The following applies: R 1 and R 2 are organyl radicals (alkyl radicals, aryl radicals, arylalkyl radicals, etc.) or hydrogen atoms. R 3 can be an organyl radical (alkyl radical, aryl radical, arylalkyl radical, etc.). |
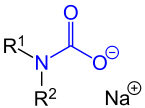
| Carbamate sodium salt - salt of carbamic acid - with the carbamate group marked in blue . The following applies: R 1 and R 2 are organyl radicals (alkyl radicals, aryl radicals, arylalkyl radicals, etc.) or hydrogen atoms. The sodium cation is just one example; other cations can also serve as a counterion to the carbamate anion. |
Carbamates are salts and esters of carbamic acids (R 2 N – COOH). The esters are more commonly referred to as urethanes . Also polyurethanes are among the carbamates.
Manufacturing
Carbamates can be prepared from isocyanates and alcohols by an addition reaction , where R 1 and R 2 organyl groups , such as. B. Alkyl groups or aryl groups are:
Alternatively, carbamates can be produced by reacting chloroformic acid esters or carbonic acid esters with amines.
In rare cases a production from an amine and CO 2 may succeed:
- R 2 NH + CO 2 → R 2 NCO 2 H
In general, however, carbamic acids are unstable and decarboxylate.
properties
Urethanes are mostly solid materials that crystallize well and are therefore used as identification preparations in chemical analysis.
The toxicity of some carbamates (e.g. bendiocarb ) is the same as that of phosphoric acid derivatives ( phosphoric acid esters ) used as pesticides and is based on their neurotoxic effect. Persistence in the environment is 1 to 12 weeks. Carbamates are more stable to hydrolysis than esters of orthophosphoric acid .
use
Since the 1950s, carbamates have been used primarily as insecticides , fungicides and herbicides in agriculture. The worldwide consumption of carbamates in 1995 was around 30,000 tons. Ammonium carbamate is important as an intermediate product in the industrial production of urea . Some carbamates were previously used as sleep aids . In organic synthesis , carbamates are used as protective groups for amines because of their stability towards base-catalyzed hydrolysis .
Carbamate-analogous groups of substances with heteroatoms
By replacing one or both oxygen atoms in the molecule, one arrives at the heteroanalogous compounds of the carbamates (R 1 to R 4 = aliphatic or aromatic radicals or hydrogen).
Thiolocarbamates
(syn .: thiolourethane )Thionocarbamates
(Syn .: Thionourethane )Dithiocarbamates
(syn .: dithiourethane)
Individual evidence
- ^ Siegfried Hauptmann : Organic Chemistry , VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 325. ISBN 3-342-00280-8 .
- ↑ Entry on protection groups. In: Römpp Online . Georg Thieme Verlag, accessed on September 5, 2013.
- ↑ Matthys J. Janssen: Thiolo, Thiono, and Dithio Acids and Esters in: The Chemistry of Carboxylic Acids and Their Esters (1969) Chap. 15th