Protecting group


A protecting group ( English protecting group - therefore often referred to as common abbreviation in formula schemes PG ) is in chemistry a substituent which, during a more complicated, multi-step chemical synthesis in a molecule is introduced to a specific functional group to protect temporarily and so an undesirable reaction to prevent this group. After the desired reaction has been carried out elsewhere on the molecule, the protective group is split off again. For many functional groups several possible protective groups are known which differ in their stability and the conditions for their cleavage.
In the synthesis of special classes of compounds with repeating functional groups - usually biomolecules such as peptides , oligosaccharides or nucleotides - standard sets of protective groups have become established. Protecting groups have become an important tool in the synthesis of complex compounds today.
The requirements for a protection group are quite high. This includes that it can be introduced specifically to a functional group with very good yield and that it must also be able to be split off again under mild conditions. It should be possible to standardize the reaction conditions for both steps. In addition, the protective group must be stable under as many reaction conditions as possible. If possible, the resulting reaction products should be easily separable, and optimally the protective group reagent is also inexpensive. The broader the experience with a protective group, the better the predictability of the reactivity of the protective group.
history
The history of protective group technology is inextricably linked with the targeted use of different starting compounds for the synthesis of a target molecule. The early protective groups were usually based on the fact that the starting compound was chosen so that a reactive functional group was blocked by a residue and was therefore unreactive. So were z. B. anisoles instead of phenols or esters instead of free alcohol groups . It was only with the targeted synthesis of increasingly complex compounds that emerged from the beginning of the 20th century that protective group technology became really important. From around 1960 onwards, considerable research expenditure began to be invested in the chemistry of the protective groups. During this time chemists began to synthesize increasingly complex natural products . Particularly noteworthy are the previous work of Nobel Prize winners Robert B. Woodward , Elias J. Corey and Albert Eschenmoser , who pioneered the synthesis of complex natural substances.
Today there are a large number of protective groups, which are summarized in monographs with regard to their properties. In addition to established protective groups, there are many exotic protective groups that were only developed for a synthesis or a very special area.
Requirements for a protection group
The introduction and removal of protective groups do not represent productive reactions in a sequence of synthetic steps; their product does not come closer to the desired end product of the synthesis. For this reason, high requirements are often placed on protecting group reactions in terms of price, yield and development effort for the reaction.
The following characteristics have emerged as the basic requirements for a good protection group:
- The reagent must be commercially available and inexpensive or easy to manufacture
- The protective group must be simple, specific and capable of being introduced in high yields
- It must be stable to the greatest possible number of reaction conditions and work-up and purification methods
- It must be specific, highly selective and capable of being split off in high yields. It should be possible to standardize the conditions.
- It must not form a new stereocenter or a diastereotopic center
- It should be easily recognizable in NMR spectra and interfere with as little signal overlap as possible
The high selectivity of the cleavage is a very important aspect, because different functional groups often have to be protected and deprotected independently of one another. Ideally, only one of many protective groups is affected by the cleavage process. The behavior of protective groups in practice cannot always be correctly predicted on the basis of the literature, especially if several different protective groups are used in one molecule. Therefore, in some cases, despite a great deal of experience, considerable development work must still be done during a synthesis for both the introduction and the splitting off.
Orthogonality of protecting groups

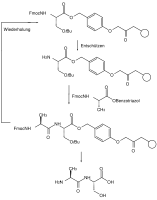
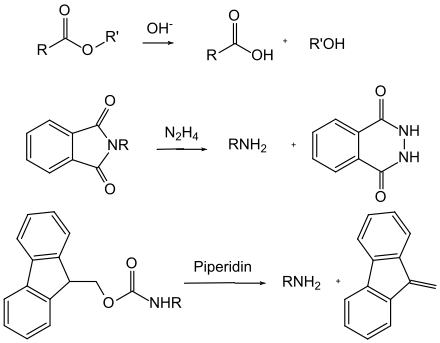
Orthogonality of protective groups means that when several protective groups of different types are used, each protective group can be split off individually and in any order on the basis of the various splitting off reagents without any of the other protective groups being attacked. In the example of the protected amino acid tyrosine shown , the benzyl ester can be split hydrogenolytically , the fluorenylmethyleneoxy group (Fmoc) by bases (e.g. piperidine ) and the phenolic tert-butyl ether with acids (e.g. trifluoroacetic acid ).
A widespread example of this application is Fmoc peptide synthesis, which has gained great importance both in solution and on the solid phase. The protective groups in the solid phase synthesis must be standardized with regard to the reaction conditions such as reaction time, temperature and reagents so that they can be carried out by an automatic device and yields of well over 99% can be achieved, since otherwise the separation of the resulting mixture of reaction products is practically impossible .
Another important application of orthogonal protecting groups is in carbohydrate chemistry. Since carbohydrates have hydroxyl groups with very similar reactivity, the protection or deprotection of individual hydroxyl groups must be possible for a targeted synthetic conversion. The synthesis of nucleotides represents a similar case . On the one hand, there is the problem (as with peptide synthesis) that the molecules are vectorial. On the other hand, there is also the problem of carbohydrate chemistry with the sugar residue of the ribose in the synthesis of RNA molecules.
But also in the synthesis of complex natural substances or active ingredients with many functional groups one is dependent on the orthogonality of the protective groups.
Lability or splitting off of protective groups
In the case of protective groups, various reaction conditions have been established which correspond to the principle of orthogonality, under which protective groups are split off. One can roughly differentiate between the following cleavage conditions:
- Acid-labile protecting groups
- Base-labile protecting groups
- Fluoride-labile protecting groups
- Enzyme-labile protecting groups
- Reduction- labile protecting groups
- Oxidation- labile protecting groups
- Protective groups that are split by heavy metal salts or their complexes
- Photolabile protecting groups
- Two-stage protecting groups
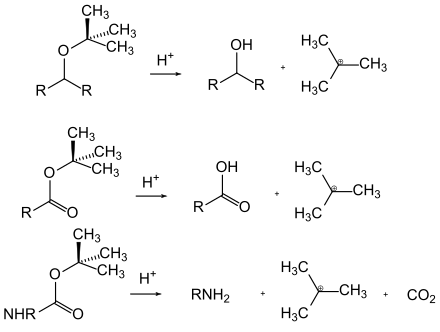
Acid-labile protective groups can be split off through the action of acids. The driving force here is often the formation of a relatively stable carbocation or an acid-catalyzed equilibrium that is on the side of the free functional group. Examples of acid-labile protective groups are the tert-butyl esters, ethers and carbamates, which form stable cations, and the acetals , in which the acid-catalyzed equilibrium is on the side of the corresponding aldehydes or ketones in the presence of water.
In the case of the base-labile protective groups, a mechanistical distinction can be made between basic hydrolysis and base-induced β-elimination . Carboxylic acid esters (with the exception of the tert-butyl esters) are attacked nucleophilically by hydroxide ions and thus split hydrolytically. Amides, on the other hand, are seldom split in this way because they require very harsh conditions. The phthaloyl group is an exception here , as it is cleaved with hydrazine under very mild conditions. A cascade of reactions occurs during β-elimination: First, a proton is split off by the base and a carbanion is formed. The protective group is then cleaved by a suitable leaving group to form a vinyl compound . In the latter case, the Fmoc group is particularly important.
Fluoride ions form a very stable bond with silicon . Therefore, the silicon protective groups are almost without exception cleaved by fluoride ions. Depending on the type of counterion or the cleavage reagent, however, different silicon protective groups can also be selectively cleaved depending on the steric hindrance of the silicon atom. The advantage of fluoride-labile protective groups is that no other protective group is attacked under the cleavage conditions.
Esters can often be cleaved by enzymes such as lipases . Since enzymes work at a pH value between 5 and 9 and at moderate temperatures of around 30–40 ° C and are also very selective with regard to the carboxylic acid, this method is a rarely used but very attractive method for splitting protecting groups .
Benzyl groups can be cleaved reductively by catalytic hydrogenation . Benzyl groups are used as ethers, esters, urethanes, carbonates or acetals and are used to protect alcohols, carboxylic acids, amines and diols.
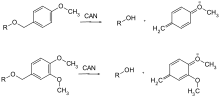
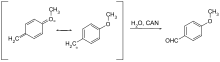
Only a few protective groups that can be removed by oxidation are used. This is usually methoxybenzyl ether. They can be cleaved with cerium (IV) ammonium nitrate (CAN) or dichlorodicyanobenzoquinone (DDQ) via a quinomethine .
The double bond of an allyl radical can be isomerized to the vinyl compound by platinum group elements (such as palladium , iridium or platinum ) . The enol ethers thus obtained in the case of protected alcohols or enamines in the case of protected amines can easily be hydrolyzed under acidic conditions.
Photolabile protective groups contain a chromophore that can be activated by irradiation with a suitable wavelength and thus split off. The o -nitrobenzyl group (ONB) is listed here as an example .
The two-stage protective groups are a special form of protective groups . These are characterized by high stability, since the protective group must first be converted into a cleavable group by chemical transformation. However, this type of protective group is rarely used, since an additional activation step is necessary here, which extends the synthesis by a further stage.
Functional groups
Amines
By far the greatest variety of protecting groups is available for the amino function . This is due on the one hand to the fact that amines are of particular importance in peptide synthesis , but also to their properties: On the one hand, they are quite potent nucleophiles , but also relatively strong bases . These properties have led to the development of new protective groups for amines.
Many protecting groups for amines are based on carbamates . These can easily be introduced in the form of carboxylic acid chlorides. They get their driving force in the split from the formation of the very stable carbon dioxide molecule. Various cleavage options have been developed based on different residues on the carbamate. The most commonly used carbamates are the tert -butyloxycarbonyl, benzyloxycarbonyl, fluorenylmethyleneoxycarbonyl and allyloxycarbonyl compounds.
| rest | formula | Surname | abbreviation | cleavage |
|---|---|---|---|---|
| tert -butyl | 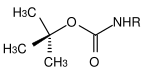 |
tert -Butyloxycarbonyl | Boc | angry; Trifluoroacetic acid (TFA) pure or as a solution in dichloromethane , 3 M hydrochloric acid in ethyl acetate or 10% sulfuric acid in dioxane |
| Benzyl | 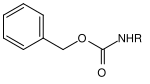 |
Benzyloxycarbonyl | Cbz or Z | hydrogenolytic; Hydrogen and palladium on activated carbon , lithium or sodium in liquid ammonia |
| Fluorenylmethylene | 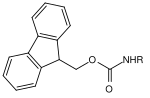 |
Fluorenylmethylene oxycarbonyl | Fmoc | basic; 20–50% piperidine in dimethylformamide (DMF) or N-methyl-2-pyrrolidone , 50% morpholine in DMF for sensitive glycopeptides |
| Allyl | 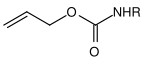 |
Allyloxycarbonyl | Alloc | transition metal catalyzed cleavage; Metals such as palladium (0) or nickel (0) complexes |
In addition to the carbamates, a number of other N- acyl derivatives are important as protective groups, but are nowhere near as widespread. These include, for example, the phthalimides , which are accessible either by reacting the primary amines with phthalic anhydride or by building up the amino group via a Gabriel synthesis . The phthalimides are normally split by hydrazine hydrate or sodium boronate . Trifluoroacetamides are extremely easy to saponify in the base , which is why the acetamides obtained by the reaction with trifluoroacetic anhydride are occasionally used as a protective group for amines.
In the case of indoles , pyrrole and imidazoles , i.e. heterocyclic compounds, the N- sulfonyl derivatives are used as protective groups. In the case of normal amines, this protective group is often too stable. They are represented here by sulfonation with phenylsulfonyl chloride and the deprotonated heterocycle. The cleavage takes place by basic hydrolysis. N- acyl derivatives of primary and secondary amines are relatively easily accessible by reacting the amines with an aryl sulfonic acid chloride, but they can only be obtained with difficulty, e.g. B. under the conditions of a Birch reduction ( sodium in liquid ammonia ) or by reaction with sodium naphthalide .
Among the N -alkyl derivatives, the representable by alkylation or reductive alkylation have N -benzyl derivatives of some importance. As with the Cbz group, the cleavage takes place reductively and normally by catalytic hydrogenation or by Birch reduction. Here, N- alkylamines have the decisive disadvantage compared to carbamates or amides that the basic nitrogen is retained.
Alcohols
The classic protective group for alcohols are carboxylic acid esters . The esters of precursors are often commercially available or can easily be obtained by reacting the alcohols with the acid chlorides or anhydrides by a Schotten-Baumann reaction or by transesterification . The esters are usually cleaved by reacting them with nucleophiles such as alkali hydroxides, alkali alcoholates or lithium or magnesium organic compounds ; alternatively also reductive by reaction with complex hydrides such as lithium aluminum hydride . The reactivity of the esters towards nucleophilic attack decreases with the steric hindrance of the carboxylic acid in the following order:
- Chloroacetyl> acetyl> benzoyl> pivaloyl
The reactivity of the alcohols also decreases with the increasing steric hindrance of the alcohols:
The most important esters that are commonly used as protective groups are the acetic acid esters , the benzoic acid esters and the pivalic acid esters , since these can be split off from one another in a differentiated manner according to the stated reactivities.
The most important protective groups of alcohols and phenols include the very well investigated and documented trisubstituted silyl ethers . The silicon carries both alkyl and aryl groups as organic radicals. This type of protective group has the advantage that it can be moderated very easily with regard to the introduction and especially with regard to the cleavage. These ethers are produced either in a Williamson ether synthesis from the chlorosilane and an alcoholate ion or through the use of activating reagents such as imidazole .
For purely analytical purposes, e.g. B. to make a carbohydrate volatile and to be able to detect it with the help of GC-MS , there are commercially available reaction kits. Silyl ethers are generally sensitive to acids and fluoride ions. The latter is mostly used to split them. However, the commercial prices of the chlorosilanes vary greatly depending on the substitution. The cheapest chlorosilane is the chlorotrimethylsilane (TMS-Cl), which is a by-product of the silicone production according to Rochow and Müller . Another common source of the trimethylsilyl group is hexamethyldisilazane (HMDS). However, the trimethylsilyl ethers are also extremely sensitive to acidic hydrolysis (for example silica gel is sufficient as a proton donor) and are therefore rarely used as a protective group today.
| Surname | formula | abbreviation | cleavage |
|---|---|---|---|
| Trimethylsilyl | 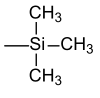 |
TMS | Potassium fluoride , acetic acid or potassium carbonate in methanol |
| Triethylsilyl |  |
TES | 10-100 times more stable than a TMS group; Trifluoroacetic acid in water / tetrahydrofuran , acetic acid in water / tetrahydrofuran, hydrofluoric acid , pyridinium hydrofluoride in pyridine |
| tert -butyldimethylsilyl |  |
TBS, TBDMS | Acetic acid in tetrahydrofuran / water, pyridinium tosylate in methanol, trifluoroacetic acid in water, hydrofluoric acid in acetonitrile , pyridinium hydrofluoride in tetrahydrofuran, tetrabutylammonium fluoride in THF |
| Triisopropylsilyl |  |
TIPS | Under the same conditions as TBS but longer reaction times; Tetrabutylammonium fluoride in tetrahydrofuran, hydrofluoric acid in acetonitrile, pyridinium hydrofluoride in tetrahydrofuran. |
| tert -Butyldiphenylsilyl |  |
TBDPS | Under the same conditions as TBS but longer reaction times (100–250 times slower than TBS and 5–10 times slower than TIPS); Tetrabutylammonium fluoride in tetrahydrofuran, hydrofluoric acid in acetonitrile, pyridinium hydrofluoride in tetrahydrofuran |
Another class of protecting groups for alcohols are the alkyl ethers. Here, too, there are various and orthogonal possibilities to split the ethers. Aliphatic methoxy ethers are difficult to cleave and under drastic conditions, so that they are generally only used with phenols.
| Surname | formula | abbreviation | cleavage |
|---|---|---|---|
| methyl | Me | Usually only used for phenols; Iodotrimethylsilane in chloroform , dichloromethane or acetonitrile, boron tribromide or boron trichloride in dichloromethane, Lewis acids (aluminum chloride, boron trifluoride in the presence of thiols) | |
| Benzyl |  |
Bn | reductive; Catalytic hydrogenation (palladium on activated carbon, Raney nickel or rhodium on aluminum oxide as a catalyst) |
| p -methoxybenzyl | PMB, MPM | oxidative; DDQ (dichlorodicyanoquinone) in dichloromethane, ceric ammonium chloride in water | |
| 3,4-dimethoxbenzyl | 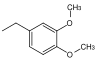 |
DMB, DMPM | like PMB oxidative; DDQ (dichlorodicyanoquinone) in dichloromethane, ceric ammonium chloride in water |
| Triphenylmethyl (trityl) | 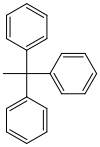 |
Tr | angry; Formic acid in ether or water, 80% acetic acid, 1 M hydrochloric acid |
| tert -butyl |  |
angry; anhydrous trifluoroacetic acid, hydrobromic acid / acetic acid, 4N hydrochloric acid | |
| Allyl | Potassium tert -butanolate, palladium on activated carbon, DABCO in methanol, various platinum element complexes - then acidic processing. | ||
| Allyloxycarbonyl | Alloc | Like allyl; Potassium tert -butanolate, palladium on activated carbon, DABCO in methanol, various platinum element complexes - then acidic processing | |
| Methoxymethyl | MOM | Angry; 6 M hydrochloric acid in tetrahydrofuran / water | |
| Methylthiomethyl | MTM | Mercury (II) chloride / calcium carbonate in acetonitrile / water, silver nitrate in tetrahydrofuran / water | |
| (2-methoxyethoxy) methyl | MEM | Aqueous hydrobromic acid in tetrahydrofuran, zinc bromide in dichloromethane | |
| Benzyloxymethyl | BOM | Comparable to the stability of MOM, MEM and SEM; Reductive; Sodium in liquid ammonia, catalytic hydrogenation (palladium hydroxide on activated carbon), Raney nickel in ethanol | |
| β- (trimethylsilyl) ethoxymethyl | 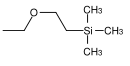 |
SEM | More unstable than MEM and MOM towards acid hydrolysis; 0.1 M hydrochloric acid in methanol, concentrated hydrofluoric acid in acetonitrile, boron trifluoride etherate in dichloromethane, tetrabutylammonium fluoride in HMPT ( hexamethylphosphoric acid triamide ) or in tetrahydrofuran |
| Tetrahydropyranyl |  |
THP | Acetic acid in tetrahydrofuran / water, p -toluenesulfonic acid in methanol |
1,2-diols
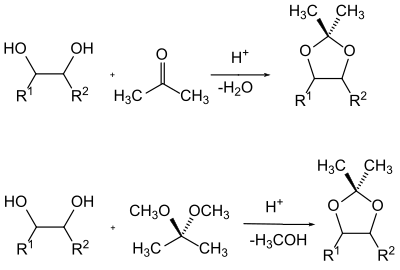
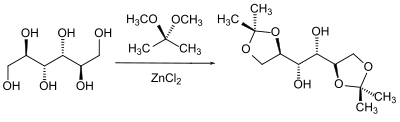
A special class of alcohols in protecting group chemistry are the 1,2-diols ( glycols ). The neighboring position of two hydroxyl groups can e.g. B. in the case of sugars , take advantage of the fact that both hydroxyl groups are protected as acetals depending on one another . The benzylidene , isopropylidene and cyclohexylidene or cyclopentylidene acetals are common here .
The acetals are generally produced by shifting the equilibrium of a mixture of the glycol with the carbonyl component by removing the water of reaction or by transacetalization with a simple acetal and removing the alcohol formed from the reaction mixture.
In sugar chemistry in particular, the different positions of the hydroxyl groups with respect to one another are used to protect them selectively in a certain stereochemical dependency. In addition to the other possible combinations, the two adjacent hydroxyl groups which form the most stable conformation react preferentially with one another.
Acetals can in principle be split again in aqueous acidic solvents. The benzylidene protective group, which can also be split reductively, is a special case here. This is done either by catalytic hydrogenation or by the hydride donor diisobutylaluminum hydride (DIBAL). However, the cleavage by DIBAL only deprotects one alcohol group, since the benzyl residue remains on the second and more sterically hindered hydroxy group than benzyl ether.
Carbonyl groups
Carbonyl groups are primarily at risk from nucleophilic attacks such as Grignard reagents or from hydride ions. Aldehydes can also be oxidized to carboxylic acids. But also unwanted reactions caused by acid- and base-catalyzed reactions of the carbonyl group such. B. Aldol reactions can be prevented by a suitable protective group.
The most common protective groups for carbonyl groups are acetals and especially cyclic acetals with diols. In addition, cyclic acetals with 1,2-hydroxythiols or dithioglycols are also used - the so-called O , S or S , S acetals.
In principle, the same applies to acetals as a protective group for carbonyl compounds as to acetals as a protective group for 1,2-diols. Both the production and the cleavage are naturally identical. However, with acetals as a protective group, the process of transacetalization plays a subordinate role, and they are usually produced from the glycols by splitting off water. More modern variants also use glycols, in which the hydroxy hydrogen atoms have been replaced by a trimethylsilyl group. Normally, simple glycols such as ethylene glycol or 1,3-propanediol are used as diols for the acetals.
Acetals can be cleaved under acidic aqueous conditions. The mineral acids are used as acids. The cosolvent is often acetone , which is used as a solubilizer. As a non-acidic elimination method, a palladium (II) chloride- acetonitrile complex in acetone or iron (III) chloride on silica gel absorbed in chloroform can be used.
Cyclic acetals are much more stable to acid hydrolysis than acyclic acetals. Therefore, acyclic acetals are used almost exclusively if a very mild cleavage is necessary or if two different protected carbonyl groups have to be differentiated with regard to their release.
However, in addition to their sole function as a protective group, acetals are also used as a chiral auxiliary reagent. So acetals of chiral glycols such as. B. derivatives of tartaric acid can be opened asymmetrically with high selectivity. This enables the creation of new centers of chirality.
In addition to the O , O -acetals, the S , O- and S , S -acetals also have an, albeit lesser, importance as a carbonyl protective group. Thiols , which have to be used to produce these acetals, have a very unpleasant odor and are poisonous, which limits their use very much. Thioacetals and the mixed S , O -acetals are, compared to the pure O , O -acetals, much more stable towards acid hydrolysis. This enables the selective cleavage of these carbonyl groups protected by sulfur in the presence .
The S , S acetals are normally produced analogously to the O , O acetals by acid catalysis from the dithiols and the carbonyl component. Due to the great stability of the thioacetals, the equilibrium is on the side of the acetals. In contrast to the O , O acetals, no water of reaction has to be removed in order to shift the equilibrium.
S , O acetals are hydrolysed 10,000 times faster than the corresponding S , S acetals. They are produced in analogy to these from the thioalcohols. Their cleavage also takes place under comparable conditions and primarily through mercury (II) compounds in aqueous acetonitrile.
Temporary protection of the carbonyl group in the presence of ketones as hemiaminal anions has been described for aldehydes . This exploits the fact that aldehydes have a much higher carbonyl activity than ketones and that many addition reactions are reversible.
Carboxy groups
The most important protecting groups for carboxy groups are the esters of various alcohols. Ortho-esters and oxazolines are also used, but of minor importance. There are basically different methods for the production of carboxylic acid esters:
- Direct esterification of carboxylic acids and alcohol components. Because of the unfavorable equilibrium in the reaction between alcohols and carboxylic acids, equilibrium must either be achieved by removing the water of reaction or by working with large excesses of alcohol. To do this, however, the alcohol has to be very cheap. This reaction is acid-catalyzed (sulfuric acid, p -toluenesulfonic acid or acidic ion exchangers are the most common esterification catalysts).
- The reaction of acid anhydrides or acid chlorides with alcohols in the presence of auxiliary bases. Pyridine , diisopropylethylamine or triethylamine are often used here as auxiliary bases . This reaction can be catalyzed with 4- N , N- dimethylaminopyridine , which increases the reaction rate by a factor of 10 4 compared to pure pyridine . Compared to direct esterification, these methods are carried out under very mild conditions.
- The reaction of carboxylic acid salts with alkyl halides is another method for the preparation of carboxylic acid esters.
- The reaction of carboxylic acids with isobutene is a gentle method for making tert-butyl esters. Here isobutene is reacted with the carboxylic acid in the presence of a strong acid such as sulfuric acid.
- The reaction of carboxylic acids with diazoalkanes is a very gentle and quantitative method to make esters. Due to the poor accessibility of complex diazoalkanes, however, it is mostly only used for the production of methyl and benzhydril esters.
In addition to these classic methods of esterification, other and more modern methods have been developed for special reactions.
- The activation of the carboxylic acid with dicyclohexylcarbodiimide and reaction of the O -acylisourea thus obtained with the alcohol component in the presence of 4- N , N- dimethylaminopyridine ( Steglich esterification ).
- Activation of the carboxylic acid by producing a mixed anhydride with 2,4,6-trichlorobenzoic acid by reacting the carboxylic acid with benzoyl chloride in the presence of 4- N , N- dimethylaminopyridine and triethylamine. The mixed anhydride is produced in situ and immediately reacted further with the alcohol component ( Yamaguchi esterification ).
- The activation of the alcohol component through the reaction under Mitsunobu conditions with diethylazodicarboxylate and triphenylphosphine and subsequent reaction in situ with the carboxylic acid ( Mitsunobu esterification ).
Various groups can serve as the alcohol component. However, the methyl, tert- butyl, benzyl and allyl esters are very common here . In addition, there are a number of protective groups, which are derived from the ether protective groups of the hydroxyl groups. However, the specific cleavage conditions are often very similar. In principle, any ester can be hydrolyzed in the presence of hydroxide ions in an aqueous-alcoholic solution. With more sensitive substrates, however, lithium hydroxide in tetrahydrofuran and in the presence of methanol is often used . The same rules naturally apply to the tendency towards hydrolysis as to esters as alcohol protecting groups.
| Surname | formula | abbreviation | cleavage | Special manufacture |
|---|---|---|---|---|
| methyl | Me | nucleophilic-alkaline by metal hydroxides or carbonates in aqueous methanol or tetrahydrofuran, alkali metal halides in moist aprotic solvents such as dimethyl sulfoxide, N , N -dimethylformamide in the heat, enzymatically z. B. by pig liver esterase | Diazomethane in diethyl ether, cesium carbonate and methyl iodide in N , N -dimethylformamide, methanol and catalytic trimethylsilyl chloride | |
| tert -butyl |  |
tert -Bu | angry; Trifluoroacetic acid (pure or in dichloromethane), formic acid, p -toluenesulfonic acid | Isobutene in dioxane and catalytic sulfuric acid |
| Benzyl |  |
Bn | hydrogenolytic; Hydrogen / palladium on activated carbon | |
| Benzhydryl | 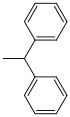 |
hydrogenolytic; Hydrogen / palladium on activated carbon (very easy to split) | ||
| Allyl | Allyl | analogous to ethers; Potassium tert- butanolate, palladium on activated carbon, DABCO (1,4-diazabicyclo [2.2.2] octane) in methanol, various platinum element complexes - then acidic processing |
Alkenes
Alkenes are and rarely need to be protected by a protective group. As a rule, they are only affected by electrophilic attacks, isomerization and, during catalytic hydrogenation, of undesired side reactions. Basically, two protective groups are known for alkenes:
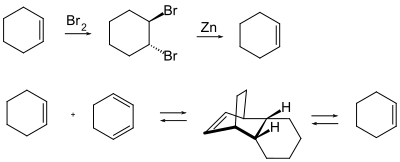
- Temporary halogenation with bromine to form the trans -1,2-dibromoalkyl compound: The alkene is regenerated by restoring the conformation with elemental zinc or with titanocene dichloride .
- Protection through a Diels-Alder reaction : the reaction of an alkene with a diene leads to a cyclic alkene, which is at risk from electrophilic attack in a similar way to the original alkene. The diene serving as a protective group is split off thermally, since a Diels-Alder reaction is a reversible or equilibrium reaction.
Alkynes
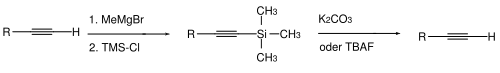
Two types of protective groups are also known for alkynes. With terminal alkynes it is sometimes necessary to mask the acidic hydrogen atom. This is usually done by deprotonation (using strong bases such as methylmagnesium bromide or butyllithium in tetrahydrofuran / dimethylsulfoxide ) and subsequent reaction with chlorotrimethylsilane to form the terminally TMS-protected alkyne. The cleavage takes place hydrolytically - with potassium carbonate in methanol - or by fluoride ions such as, for example, by means of tetrabutylammonium fluoride .
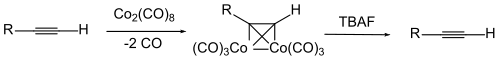
To protect the triple bond itself, a complex of the alkyne compound with dicobalt octacarbonyl is sometimes used. The cobalt is split off by oxidation.
Applications
Protecting groups are used in a wide range of synthetic organic chemistry. This applies to both laboratory syntheses and large-scale syntheses of complex active ingredients. As soon as a functional group proves to be disruptive or can be attacked undesirably, the protective group technique is used. Protecting groups are used in almost every synthesis of a complex target molecule. Since both the introduction and the cleavage of the protective groups result in both the effort and a loss of yield, it is desirable to manage without protective groups, which, however, is often difficult to achieve.
In the automated synthesis of peptides and nucleotides, protecting group chemistry is an integral part of the synthesis concept. Protecting groups are also indispensable in sugar chemistry due to the very similar hydroxyl groups in the sugar molecules.
An important example of the industrial application of protective group technology is the synthesis of ascorbic acid (vitamin C) according to Reichstein .
In order to prevent the secondary alcohols from being oxidized by potassium permanganate , they are protected by acetalization with acetone and then deprotected again after the primary hydroxyl group has been oxidized to the carboxylic acid.
A very spectacular example from the synthesis of natural products for the application of protective groups is the total synthesis of palytoxin carboxylic acid by the working group of Yoshito Kishi from 1994. Here 42 functional groups (39 hydroxyl groups, one diol, one amino group and one carboxylic acid group) had to be protected. This was done using eight different protective groups (one methyl ester, five acetate groups, 20 TBDMS ethers, nine p- methoxybenzyl ethers, four benzoates, one methyl hemiacetal, one acetal with acetone and one SEM ester).
The introduction or modification of a protective group sometimes also influences the reactivity of the entire molecule. As an example, an excerpt from the synthesis of an analogue of Mitomycin C by Danishefsky is shown here.
Switching the protective group from a methyl ether to a MOM ether prevents the epoxide from opening to form the aldehyde .
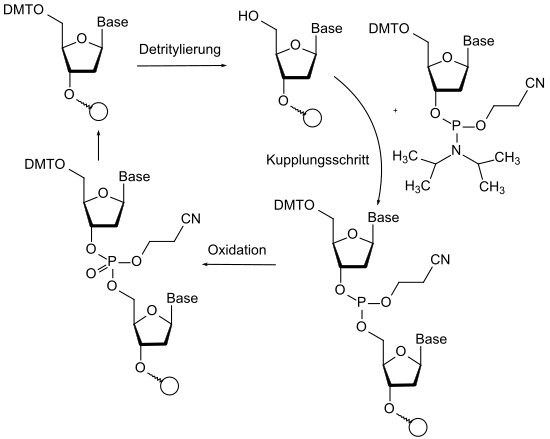
An important application of protecting group chemistry can be found in the automated synthesis of peptides and nucleosides. In the peptide synthesis by automatic synthesizers, the orthogonality of the Fmoc group (basic cleavage), the tert- butyl group (acidic cleavage) and various protective groups for functional groups in the side chain of the amino acids are used. In the automated nucleotide synthesis of DNA and RNA sequences, protective groups are used on the one hand to block functional groups, but redox chemistry also takes place on the protected atoms. The phosphorus is protected and oxidized to phosphate during the coupling cycle.
As a rule, introducing a protective group is not a problem. The difficulties lie more in their stability and selective splitting. Problems that arise in synthesis strategies with protective groups are only rarely documented in the specialist literature.
Atomic economy
Syntheses using protective groups generally have a low atom economy . Sometimes the detour of using protective groups has to be taken in order to eliminate undesired competing reactions and to achieve the desired selectivity of a synthesis. Protecting group strategies are often indispensable in the synthesis of complex structures.
The syntheses of Hapalindol U are compared as an example of a protecting group strategy compared to a synthesis free of protecting groups . While the synthesis by Hideaki Muratake from 1990 used tosyl as a protecting group, in the synthesis by Phil S. Baran from 2007 no protecting group was used. The number of synthesis steps was significantly reduced.
Hapalindol U Muratake 1990 Ts protective group synthesis (protective groups in blue .)
literature
- Philip J. Kocieński : Protecting Groups , 1st edition, Georg Thieme Verlag, Stuttgart 1994, ISBN 3-13-135601-4 .
- Peter GM Wuts, Theodora W. Greene: Green's Protective Groups in Organic Synthesis , 4th Ed., John Wiley & Sons Inc., Hoboken, New Jersey, ISBN 0-471-69754-0 .
- Michael Schelhaas, Herbert Waldmann : “Protection group strategies in organic synthesis”, in: Angewandte Chemie , 1996 , 103 , pp. 2192–2219; doi: 10.1002 / anie.19961081805 .
- Krzysztof Jarowicki, Philip Kocieński: “Protecting groups” , in: J. Chem. Soc., Perkin Trans. 1 , 1998 , pp. 4005-4037; doi: 10.1039 / A803688H .
Web links
- University of Marburg : Protective groups in synthetic organic chemistry (PDF; 284 kB)
- Organic-Reaction.com: Protecting Group
- Organic-Chemistry.org: Protecting Groups
Individual evidence
- ↑ a b Kyriacos C. Nicolaou, Erik J. Sorensen: Classics in Total Synthesis: Targets, Strategies, Methods , 1996, ISBN 3-527-29284-5 .
- ↑ a b c Kyriacos C. Nicolaou, Scott A. Snyder: Classics in Total Synthesis II , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2003, ISBN 3-527-30684-6 .
- ↑ Philip J. Kocienski: Protecting Groups , 1st edition, Georg Thieme Verlag, Stuttgart 1994, ISBN 3-13-135601-4 .
- ↑ Peter GM Wuts, Theodora W. Greene: Green's Protective Groups in Organic Synthesis , Fourth Ed. John Wiley & Sons Inc., Hoboken, New Jersey, ISBN 0-471-69754-0 .
- ↑ PJ Kocieński: Protecting Groups , pp. 245-250.
- ↑ Dietrich Spitzner, Kai Oesterreich: “Anionically Induced Domino Reactions - Synthesis of a Norpatchoulenol-Type Terpene”, in: European Journal of Organic Chemistry , 2001 , 10 ; Pp. 1883-1886; doi : 10.1002 / 1099-0690 (200105) 2001: 10 <1883 :: AID-EJOC1883> 3.0.CO; 2-M .
- ↑ a b c Weng C. Chan, Peter D. White: Fmoc Solid Phase Peptide Synthesis . Reprint 2004, Oxford University Press, ISBN 0-19-963724-5 .
- ↑ Weng C. Chan, Peter D. White: Fmoc Solid Phase Peptide Synthesis , pp. 10-12.
- ↑ Kyriacos C. Nicolaou, Eric J. Sorensen: Classics in Total Synthesis: Targets, Strategies, Methods , VCH Verlagsgesellschaft mbH, Weinheim, 1996, pp. 711-729, ISBN 3-527-29284-5 .
- ↑ Michael Schelhaas, Herbert Waldmann: “Protective group strategies in organic synthesis”, in: Angewandte Chemie , 1996 , 103 , pp. 2195–2200; doi: 10.1002 / anie.19961081805 .
- ^ VN Rajasekharan Pillai: "Photoremovable Protecting Groups in Organic Synthesis", in: Synthesis , 1980 , pp. 1–26.
- ↑ PJ Kocienski: Protecting Groups , S. 186th
- ↑ Naomi Sakai, Yasufumi Ohfune: "Total synthesis of galantin I. Acid-catalyzed cyclization of galantinic acid", in: J. Am. Chem. Soc. , 1992 , 114 , pp. 998-1010; doi: 10.1021 / ja00029a031 .
- ↑ Glenn L. Stahl, Roderich Walter, Clarck W. Smith: "General procedure for the synthesis of mono-N-acylated 1,6-diaminohexanes", in: J. Org. Chem. , 1978 , 43 , p. 2285– 2286; doi: 10.1021 / jo00405a045 .
- ↑ Houghton RA, Beckman A., Ostresh JM: Int. J. Pept. Protein Res. , 1986 , 27 , p. 653.
- ↑ PJ Kocienski: Protecting Groups , S. 195th
- ↑ Robert M. Williams, Peter J. Sinclair, Dongguan Zhai, Daimo Chen: "Practical asymmetric syntheses of α-amino acids through carbon-carbon bond constructions on electrophilic glycine templates", in: J. Am. Chem. Soc. , 1988 , 110 , pp. 1547-1557; doi: 10.1021 / ja00213a031 .
- ↑ Weng C. Chan, Peter D. White: Fmoc Solid Phase Peptide Synthesis , pp. 27-30.
- ^ Gregg B. Fields: Methods for Removing the Fmoc Group. (PDF; 663 kB) In: Michael W. Pennington, Ben M. Dunn (eds.): Peptide Synthesis Protocols Volume 35, 1995, ISBN 978-0-89603-273-6 , pp. 17-27.
- ↑ B. Liebe, H. Kunz: Solid phase synthesis of a tumor-associated sialyl-Tn antigen glycopeptide with a partial sequence from the “tandem repeat” of the MUC-1 mucin In: Angew. Chem. Vol. 109, 1997, pp. 629-631.
- ↑ ChemPep Inc .: Fmoc Solid Phase Peptide Synthesis. Retrieved November 16, 2013.
- ↑ PJ Kocienski: Protecting Groups , pages 199-201.
- ↑ John O. Osby, Michael G. Martin, Bruce Ganem: An Exceptionally Mild Deprotection of Phthalimides , in: Tetrahedron Lett. , 1984 , 25 , pp. 2093-2096; doi: 10.1016 / S0040-4039 (01) 81169-2 .
- ↑ PJ Kocienski: Protecting Groups , pages 220-227.
- ↑ P. Vouros: "Chemical derivatization in gas chromatographie-mass spectrometry", in: "Mass Spectrometrie", Degger, New York, 1979, vol. 2, p. 129.
- ↑ PJ Kocienski: Protecting Groups , S. 29th
- ↑ PJ Kocienski: Protecting Groups , S. 31st
- ↑ Tod K Jones, Robert A. Reamer, Richard Desmond, Sander G. Mills: "Chemistry of tricarbonyl hemiketals and application of Evans technology to the total synthesis of the immunosuppressant (-) - FK-506", in: J. Am. Chem. Soc. , 1990 , 112 , pp. 2998-3017; doi: 10.1021 / ja00164a023 .
- ↑ Dieter Seebach, Hak-Fun Chow, Richard FW Jackson, Marius A. Sutter, Suvit Thaisrivongs, Jürg Zimmermann: "(+) - 11,11′-Di-O-methylelaiophylidene - preparation from elaiophylin and total synthesis from (R) -3-hydroxybutyrate and (S) -malate ”, in: Liebigs Ann. Chem. , 1986 , pp. 1281-1308; doi: 10.1002 / jlac.198619860714 .
- ↑ David A. Evans, Stephen W. Kaldor, Todd K. Jones, Jon Clardy, Thomas J. Stout: "Total synthesis of the macrolide antibiotic cytovaricin", in: J. Am. Chem. Soc. , 1990 , 112 , pp. 7001-7031; doi: 10.1021 / ja00175a038 .
- ↑ James A. Marshall, Richard Sedrani: “A convergent, highly stereoselective synthesis of a C-11-C-21 subunit of the macbecins”, in: J. Org. Chem. , 1991 , 56 , pp. 5496-5498; doi: 10.1021 / jo00019a004 .
- ↑ a b James D. White, Motoji Kawasaki: "Total synthesis of (+) - latrunculin A", in: J. Am. Chem. Soc. , 1990 , 112 , pp. 4991-4993; doi: 10.1021 / ja00168a071 .
- ↑ Morris J. Robins, Vicente Samano, Mark D. Johnson: “Nucleic acid-related compounds. 58. Periodinane oxidation, selective primary deprotection, and remarkably stereoselective reduction of tert-butyldimethylsilyl-protected ribonucleosides. Synthesis of 9- (β-D-xylofuranosyl) adenine or 3'-deuterioadenosine from adenosine ”, in: J. Org. Chem. , 1990 , 55 , pp 410-412; doi: 10.1021 / jo00289a004 .
- ^ R. Roger F. Newton, Derek P. Reynolds, Colin F. Webb, Stanley M. Roberts: "A short and efficient total synthesis of (±) prostaglandin D 2 methyl ester involving a new method for the cleavage of a dimethyl- t-butylsilyl ether ”, in: J. Chem. Soc., Perkin Trans. 1 , 1981 , pp. 2055-2058; doi: 10.1039 / P19810002055 .
- ↑ Kyriacos C. Nicolaou, RA Daines, TK Chakraborty: "Total synthesis of amphoteronolide B", in: J. Am. Chem. Soc. , 1987 , 109 , pp. 2208-2210; doi: 10.1021 / ja00241a063 .
- ↑ Leo A. Paquette, Annette M. Doherty, Christopher M. Rayner: "Total synthesis of furanocembranolides. 1. Stereocontrolled preparation of key heterocyclic building blocks and assembly of a complete seco-pseudopterane framework “, in: J. Am. Chem. Soc. , 1991 , 109 , pp. 3910-3926; doi: 10.1021 / ja00036a045 .
- ↑ PJ Kocienski: Protecting Groups , p. 40
- ↑ PJ Kocieński: Protecting Groups , pp. 38-39.
- ↑ a b P.J. Kocieński: Protecting Groups , p. 43.
- ↑ JFW McOmie, DE West: 3,3′-Dihyroxybiphenyl In: Organic Syntheses . 49, 1969, p. 50, doi : 10.15227 / orgsyn.049.0050 ; Coll. Vol. 5, 1973, p. 412 ( PDF ).
- ↑ PJ Kocienski: Protecting Groups , pages 46-49.
- ↑ Yuji Oikawa, Tadao Yoshioka, Osamu Yonemitsu: "Specific removal of o-methoxybenzyl protection by DDQ oxidation", in: Tetrahedron Lett. , 1982 , 23 , pp. 885-888; doi: 10.1016 / S0040-4039 (00) 86974-9 .
- ^ Rolf Johansson, Bertil Samuelsson: “Regioselective reductive ring-opening of 4-methoxybenzylidene acetals of hexopyranosides. Access to a novel protecting group strategy. Part 1 ", in: J. Chem. Soc., Perkin Trans. 1 , 1984 , pp. 2371-2374; doi: 10.1039 / P19840002371 .
- ↑ Literature such as p -methoxybenzyl.
- ↑ Michel Bessodes, Dimitri Komiotis, Kostas Antonakis: "Rapid and selective detritylation of primary alcohols using formic acid", in: Tetrahedron Lett. , 1986 , 27 , pp. 579-580; doi: 10.1016 / S0040-4039 (00) 84045-9 .
- ↑ B. Helferich: Carbonhydr. Chem. Biochem. , 1948 , 3 , p. 79.
- ↑ ML García, J. Pascual, L. Borràs, JA Andreu, E. Fos, D. Mauleón, G. Carganico, F. Arcamone: "Synthesis of new ether glycerophospholipids structurally related to modulator", in: Tetrahedron , 1991 , 47 , Pp. 10023-10034; doi: 10.1016 / S0040-4020 (01) 96051-X .
- ↑ PJ Kocienski: Protecting Groups , pages 59-60.
- ↑ PJ Kocienski: Protecting Groups , S. 62nd
- ↑ RE Ireland, DW Norbeck: "Convergent synthesis of polyether ionophore antibiotics: the synthesis of the monensin bis (tetrahydrofuran) via the Claisen rearrangement of an ester enolate with a β-leaving group", in: J. Am. Chem. Soc. , 1985 , 107 , pp. 3279-3285; doi: 10.1021 / ja00297a038 .
- ↑ Literature see allyl.
- ^ Paul A. Wender, Carlos RD Correia: "Intramolecular photoinduced diene-diene cyaloadditions: a selective method for the synthesis of complex eight-membered rings and polyquinanes", in: J. Am. Chem. Soc. , 1987 , 109 , pp. 2523-2525; doi: 10.1021 / ja00242a053 .
- ↑ Elias J. Corey, Mark G. Bock: "Protection of primary hydroxyl groups as methylthiomethyl ethers", in: Tetrahedron Lett. , 1975 , 16 , pp. 3269-3270; doi: 10.1016 / S0040-4039 (00) 91422-9 .
- ↑ Elias J. Corey, Duy H. Hua, Bai Chuan Pan, Steven P. Seitz: "Total synthesis of aplasmomycin", in: J. Am. Chem. Soc. , 1982 , 104 , pp. 6818-6820; doi: 10.1021 / ja00388a074 .
- ↑ Serge David, Annie Thieffry, Alain Veyrières: “A mild procedure for the regiospecific benzylation and allylation of polyhydroxy compounds via their stannylene derivatives in non-polar solvents”, in: J. Chem. Soc., Perkin Trans. 1 , 1981 , Pp. 1796-1801; doi: 10.1039 / P19810001796 .
- ↑ Kaoru Fuji, Shigetoshi Nakano, Eiichi Fujita: “An Improved Method for Methoxymethylation of Alcohols under Mild Acidic Conditions”, in: Synthesis , 1975 , pp. 276-277.
- ↑ PJ Kocienski: Protecting Groups , S. 77th
- ↑ H. Nagaoka, W. Rutsch, G. Schmidt, H. Ito, MR Johnson, Y. Kishi: “Total synthesis of rifamycins. 1. Stereocontrolled synthesis of the aliphatic building block ", in: J. Am. Chem. Soc. , 1980 , 102 , pp. 7962-7965; doi: 10.1021 / ja00547a037 .
- ^ W. Clark Still, Dominick Mobilio: “Synthesis of asperdiol”, in: J. Org. Chem. , 1983 , 48 , pp. 4785-4786; doi: 10.1021 / jo00172a070 .
- ↑ Masahiro Hirama, Mitsuko Uei: “A chiral total synthesis of compactin”, in: J. Am. Chem. Soc. , 1982 , 104 , pp. 4251-4253; doi: 10.1021 / ja00379a037 .
- ↑ W. Clark Still, Shizuaki Murata, Gilbert Revial, Kazuo Yoshihara: “Synthesis of the cytotoxic germacranolide eucannabinolide”, in: J. Am. Chem. Soc. , 1983 , 105 , pp. 625-627; doi: 10.1021 / ja00341a055 .
- ↑ Robert C. Gadwood, Renee M. Lett, Jane E. Wissinger: “Total synthesis of (±) -poitediol and (±) 4-epipoitediol”, in: J. Am. Chem. Soc. , 1984 , 106 , pp. 3869-3870; doi: 10.1021 / ja00325a032 .
- ↑ Steven D. Burke, Gregory J. Pacofsky: “The ester enolate claisen rearrangement. Total synthesis of (±) -ethisolide ", in: Tetrahedron Lett. , 1986 , 27 , pp. 445-448; doi: 10.1016 / S0040-4039 (00) 85501-X .
- ↑ Toshiyuki Kan, Masaru Hashimoto, Mitsutoshi Yanagiya, Haruhisa Shirahama: “Effective deprotection of 2- (trimethylsilylethoxy) methylated alcohols (SEM ethers). Synthesis of thyrsiferyl-23 acetate “, in: Tetrahedron Lett. , 1988 , 29 , pp. 5417-5418; doi: 10.1016 / S0040-4039 (00) 82883-X .
- ↑ Joseph P. Marino, Scott L. Dax: "An efficient desilylation method for the generation of o-quinone methides: application to the synthesis of (+) - and (-) - hexahydrocannabinol", in: J. Org. Chem. , 1984 , 49 , pp. 3671-3672; doi: 10.1021 / jo00193a051 .
- ↑ Karel F. Bernady, M. Brawner Floyd, John F. Poletto, Martin J. Weiss: “Prostaglandins and congeners. 20. Synthesis of prostaglandins via conjugate addition of lithium trans-1-alkenyltrialkylalanate reagents. A novel reagent for conjugate 1,4-additions ", in: J. Org. Chem. , 1979 , 44 , pp. 1438-1447; doi: 10.1021 / jo01323a017 .
- ↑ Elias J. Corey, Haruki Niwa, Jochen Knolle: "Total synthesis of (S) -12-hydroxy-5,8,14-cis, -10-trans-eicosatetraenoic acid (Samuelsson's HETE)", in: J. Am . Chem. Soc. , 1978 , 100 , pp. 1942-1943; doi: 10.1021 / ja00474a058 .
- ^ A b P. Collins, R. Ferrier: Monosacharides - Their Chemistry and their Roles in Natural Products , Wiley, West Sussex 1995, ISBN 0-471-95343-1 .
- ↑ Christopher R. Schmid, Jerry D. Bryant: D- (R) -Glycerinaldehyde Acetonide In: Organic Syntheses . 72, 1995, p. 6, doi : 10.15227 / orgsyn.072.0006 ; Coll. Vol. 9, 1998, p. 450 ( PDF ).
- ^ András Lipták, János Imre, János Harangi, Pál Nánási, András Neszmélyi: “Chemo-, stereo- and regioselective hydrogenolysis of carbohydrate benzylidene acetals. Synthesis of benzyl ethers of benzyl α-D-, methyl β-D-mannopyranosides and benzyl α-D-rhamnopyranoside by ring cleavage of benzylidene derivatives with the LiAlH 4 -AlCl 3 reagent ", in: Tetrahedron , 1982 , 38 , p. 3721-3727; doi: 10.1016 / 0040-4020 (82) 80083-5 .
- ↑ James A. Marshall, Joseph D. Trometer, Bruce E. Blough, Thomas D. Crute: "Stereochemistry of SN2 'additions to acyclic vinyloxiranes", in J. Org. Chem. , 1988 , 53 , pp. 4274-4282 doi : 10.1021 / jo00253a020
- ↑ T. Tsunoda, M. Suzuki, R. Noyori: "A facile procedure for acetalization under aprotic conditions", in: Tetrahedron Lett. , 1980 , 21 , pp. 1357-1358; doi: 10.1016 / S0040-4039 (00) 74575-8 .
- ↑ Juji Yoshimura, Shigeomi Horito, Hiroriobu Hashimoto: "Facile Synthesis of 2,3,4,6-Tetra-O-benzyl-D-glucopyranosylidene Acetals Using Trimethylsilyl Trifluoromethanesulfonate Catalyst", in: Chem. Lett. , 1981 , 10 , pp. 375-376; doi: 10.1246 / cl.1981.375 .
- ↑ Bruce H. Lipshutz, Daniel Pollart, Joseph Monforte, Hiyoshizo Kotsuki: "Pd (II) -catalyzed acetal / ketal hydrolysis / exchange reactions", in: Tetrahedron Lett. , 1985 , 26 , pp. 705-708; doi: 10.1016 / S0040-4039 (00) 89114-5 .
- ↑ Kwan Soo Kim, Yang Heon Song, Bong Ho Lee, Chi Sun Hahn: "Efficient and selective cleavage of acetals and ketals using ferric chloride adsorbed on silica gel", in: J. Org. Chem. , 1986 , 51 , p. 404-407; doi: 10.1021 / jo00353a027 .
- ↑ PJ Kocienski: Protecting Groups , pages 167-170.
- ↑ PJ Kocieński: Protecting Groups , pp. 164-167.
- ↑ PJ Kocienski: Protecting Groups , S. 176th
- ↑ PJ Kocienski: Protecting Groups , pages 178-180.
- ↑ Samuel J. Danishefsky, Nathan B. Mantlo, Dennis S. Yamashita, Gayle. Schulte: "Concise route to the calichemicin-esperamicin series: the crystal structure of an aglycone prototype", in: J. Am. Chem. Soc. , 1988 , 110 , pp. 6890-6891; doi: 10.1021 / ja00228a051 .
- ↑ John N. Haseltine, Maria Paz Cabal, Nathan B. Mantlo, Nobuharu Iwasawa, Dennis S. Yamashita, Robert S. Coleman, Samuel J. Danishefsky, Gayle K. Schulte: “Total synthesis of calicheamicinone: new arrangements for actuation of the reductive cycloaromatization of aglycon congeners ”, in: J. Am. Chem. Soc. , 1991 , 113 , pp. 3850-3866; doi: 10.1021 / ja00010a030 .
- ↑ PJ Kocienski: Protecting Groups , S. 119th
- ↑ Satomi Niwayama: “Highly Efficient Selective Monohydrolysis of Symmetric Diesters”, in: J. Org. Chem. , 2000 , 65 , pp. 5834-5836; doi: 10.1021 / jo0001986 .
- ↑ JM Khurana, Arti Sehgal: “An efficent and convenient procedure for ester hydrolysis”, in: Org. Prep. Proced. Ind. , 1994 , 26 , pp. 580-583.
- ^ Robert V. Stevens, Albert WM Lee: "Stereochemistry of the Robinson-Schoepf reaction. A stereospecific total synthesis of the ladybug defense alkaloids precoccinelline and coccinelline ”, in: J. Am. Chem. Soc. , 1979 , 101 , pp. 7032-7035; doi: 10.1021 / ja00517a042 .
- ↑ J. Wrobel, K. Takahashi, V. Honkan, G. Lannoye, JM Cook, Steven H. Bertz: “α-Lithio ketones. 1. Stereocontrolled synthesis of (±) -modhephene via the Weiss reaction ”, in: J. Org. Chem. , 1983 , 48 , pp. 139-141; doi: 10.1021 / jo00149a034 .
- ↑ Dennis D. Keith, John A. Tortora, Roxana Yang: "Synthesis of L-2-amino-4-methoxy-trans-but-3-enoic acid", in: J. Org. Chem. , 1978 , 43 , Pp. 3711-3713; doi: 10.1021 / jo00413a016 .
- ↑ Peter Mohr, Nada Waespe-Šarčević, Christoph Tamm, Krystyna Gawronska, Jacek K. Gawronski: “A Study of Stereoselective Hydrolysis of Symmetrical Diesters with Pig Liver Esterase”, in: Helv. Chim. Acta , 1983 , 66 , pp. 2501-2511; doi: 10.1002 / hlca.19830660815 .
- ↑ Théophile Tschamber, Nada Waespe-Šarčević, Christoph Tamm: "Stereocontrolled Synthesis of an epimer of the C (19) -to-C (27) segment of rifamycin S ', in Helv. Chim. Acta , 1986 , 69 , pp. 621-625; doi: 10.1002 / hlca.19860690311 .
- ^ Yves Rubin, Carolyn B. Knobler, Francois Diederich: “Precursors to the cyclo [n] carbons: from 3,4-dialkynyl-3-cyclobutene-1,2-diones and 3,4-dialkynyl-3-cyclobutene-1 , 2-diols to cyclobutenodehydroannulenes and higher oxides of carbon ", in: J. Am. Chem. Soc. , 1990 , 112 , pp. 1607-1617; doi: 10.1021 / ja00160a047 .
- ↑ Sunggak Kim, Yong Gil Kim, Deog-il Kim: “A novel method for selective dioxolanation of ketones in the presence of aldehydes”, in: Tetrahedron Lett. , 1992 , 33 , pp. 2565-2566; doi: 10.1016 / S0040-4039 (00) 92243-3 .
- ↑ G. Bauduin, D. Bondon, Y. Pietrasanta, B. Pucci: "Reactions de transcetalisation - II: Influence des facteurs steriques et electroniques sur les energies de cetalisation", in: Tetrahedron , 1978 , 34 , pp. 3269-3274 ; doi: 10.1016 / 0040-4020 (78) 80243-9 .
- ↑ John E. McMurry, Stephen J. Isser: "Total synthesis of longifolene", in: J. Am. Chem. Soc. , 1972 , 94 , pp. 7132-7137; doi: 10.1021 / ja00775a044 .
- ↑ MP Bosch, M. Pilar Bosch, Francisco Camps, Jose Coll, Angel Guerrero, Toshio Tatsuoka, Jerrold Meinwald: "A stereoselective total synthesis of (±) -muzigadial", in: J. Org. Chem. , 1986 , 51 , Pp. 773-784; doi: 10.1021 / jo00356a002 .
- ↑ Ulrich Schmidt, Thomas Beuttler, Albrecht Lieberknecht, Helmut Griesser: “Amino acids and peptides - XXXXII. Synthesis of Chlamydocin + epi-Chlamydocin “, in: Tetrahedron Lett. , 1983 , 24 , pp. 3573-3576; doi: 10.1016 / S0040-4039 (00) 88171-X .
- ↑ Elias J. Corey, Plato A. Magriotis: "Total synthesis and absolute configuration of 7,20-diisocyanoadociane", in: J. Am. Chem. Soc. , 1987 , 109 , pp. 287-289; doi: 10.1021 / ja00235a052 .
- ↑ Elias J. Corey, Kyriacos C. Nicolaou, Takeshi Toru: "Total synthesis of (±) -vermiculine", in: J. Am. Chem. Soc. , 1975 , 97 , pp. 2287-2288; doi: 10.1021 / ja00841a058 .
- ↑ Tainejiro Hiyama, Akihiro Kanakura, Hajime Yamamoto, Hitosi Nozaki: “General Route to α, β-unsaturated Aldehydes of Homoterpenoid and Terpenoid Structure. Sythesis of JH-II and β-Sinensal “, in: Tetrahedron Lett. , 1978 , 19 , pp. 3051-3054; doi: 10.1016 / S0040-4039 (01) 94936-6 .
- ↑ F. Huet, A. Lechevallier, M. Pellet, JM Conia: “Wet Silica Gel; A Convenient Reagent for Deacetalization ", in: Synthesis , 1978 , pp. 63-64.
- ↑ F. Zymalkokowski: Catalytic Hydrogenation , Ferdinand Enke Verlag, Stuttgart 1965, pp. 127-133.
- ↑ PJ Kocienski: Protecting Groups , S. 136th
- ↑ PJ Kocienski: Protecting Groups , pages 139-142.
- ↑ Ahmed M. Tafesh, Jens Weiguny: "A Review of the Selective Catalytic Reduction of Aromatic Nitro Compounds into Aromatic Amines, Isocyanates, Carbamates, and Ureas Using CO", in: Chem. Rev. , 1996 , 96 , p. 2035– 2052; doi: 10.1021 / cr950083f .
- ^ Evan L. Allred, Boyd R. Beck, Kent J. Voorhees: “Formation of carbon-carbon double bonds by the reaction of vicinal dihalides with sodium in ammonia”, in: J. Org. Chem. , 1974 , 39 , p 1426-1427; doi: 10.1021 / jo00926a024 .
- ↑ Timothy S. Butcher, Feng Zhou, Michael R. Detty: “Debrominations of vic-Dibromides with Diorganotellurides. 1. Stereoselectivity, Relative Rates, and Mechanistic Implications ", in: J. Org. Chem. , 1998 , 63 , pp. 169-176; doi: 10.1021 / jo9713363 .
- ^ CJ Li, David N. Harpp: "Bis (triphenylstanyl) telluride a mild and selective reagent for telluration and debromination", in: Tetrahedron Lett. , 1990 , 31 , pp. 6291-6293; doi: 10.1016 / S0040-4039 (00) 97045-X .
- ↑ Corrado Malanga, Serena Mannucci, Luciano Lardicci: “Carbon-halogen bond activation by nickel catalyst: Synthesis of alkenes, from 1,2-dihalides”, in: Tetrahedron , 1998 , 54 , pp. 1021-1028; doi: 10.1016 / S0040-4020 (97) 10203-4 .
- ↑ Byung Woo Yoo, Seo Hee Kim, Jun Ho Kim: “A Mild, Efficient, and Selective Debromination of vic-Dibromides to Alkenes with Cp 2 TiCl 2 / Ga System”, in: Bull. Korean Chem. Soc. , 2010 , 31 , pp. 2757-2758; doi: 10.5012 / bkcs.2010.31.10.2757 .
- ^ Antonius JH Klunder, Jie Zhu, Binne Zwanenburg: "The Concept of Transient Chirality in the Stereoselective Synthesis of Functionalized Cycloalkenes Applying the Retro-Diels-Alder Methodology", in: Chem. Rev. , 1999 , 99 , pp. 1163-1190 ; doi: 10.1021 / cr9803840 .
- ↑ Hideyuki Tanaka, Takashi Kamikubo, Naoyuki Yoshida, Hideki Sakagami, Takahiko Taniguchi, Kunio Ogasawara: "Enantio- and Diastereocontrolled Synthesis of (-) - Iridolactone and (+) - Pedicularis-lactone", in: Org. Lett. , 2001 , 3 , pp. 679-681; doi: 10.1021 / ol0070029 .
- ↑ Martin Banwell, David Hockless, Bevyn Jarrott, Brian Kelly, Andrew Knill, Robert Longmore, Gregory Simpson: "Chemoenzymatic approaches to the decahydro-as-indacene cores associated with the spinosyn class of insecticide", in: J. Chem. Soc. , Perkin Trans. 1 , 2000 , pp. 3555-3558; doi: 10.1039 / b006759h .
- ↑ Wenzel E. Davidsohn, Malcolm C. Henry: "Organometallic Acetylenes of the Main Groups III-V", in: Chem. Rev. , 1967 , 67 , pp. 73-106; doi: 10.1021 / cr60245a003 .
- ↑ Barry J. Teobald: “The Nicholas reaction: the use of dicobalt hexacarbonyl-stabilized propargylic cations in synthesis”, in: Tetrahedron , 2002 , 58 , pp. 4133-4170; doi: 10.1016 / S0040-4020 (02) 00315-0 .
- ↑ Kenneth M. Nicholas, R. Pettit: "An alkyne protection group", in: Tetrahedron Lett. , 1971 , 37 , pp. 3475-3478; doi: 10.1016 / S0040-4039 (01) 97209-0 .
- ↑ Richard E. Connor, Kenneth M. Nicholas: "Isolation, characterization, and stability of α - [(ethynyl) dicobalt hexacarbonyl] carbonium ions", in: J. Organomet. Chem. , 1977 , 125 , C45-C48; doi: 10.1016 / S0022-328X (00) 89454-1 .
- ↑ Rosa F. Lockwood, Kenneth M. Nicholas: “Transition metal-stabilized carbenium ions as synthetic intermediates. I. α - [(alkynyl) dicobalt hexacarbonyl] carbenium ions as propargylating agents ”, in: Tetrahedron Lett. , 1977 , pp. 4163-4166; doi: 10.1016 / S0040-4039 (01) 83455-9 .
- ^ KM Nicholas, R. Pettit: "On the stability of α- (alkynyl) dicobalt hexacarbonyl carbonium ions", in: J. Organomet. Chem. , 1972 , 44 , C21-C24; doi: 10.1016 / 0022-328X (72) 80037-8 .
- ↑ T. Reichstein, A. Grüssner: "A productive synthesis of L-ascorbic acid (C vitamin)", in: Helv. Chim. Acta , 1934 , 17 , pp. 311-328; doi: 10.1002 / hlca.19340170136 .
- ^ KC Nicolaou, EJ Sorensen: Classics in Total Synthesis: Targets, Strategies, Methods , VCH Verlagsgesellschaft mbH, Weinheim 1996, pp. 711-729, ISBN 3-527-29284-5 .
- ↑ Peter GM Wuts, Theodora W. Greene: Green's Protective Groups in Organic Synthesis , 4th Ed., John Wiley & Sons Inc., Hoboken, New Jersey, pp. 10-13; ISBN 0-471-69754-0 .
- ↑ JM McClure, Samuel J. Danishefsky: "A novel Heck arylation reaction: rapid access to congeners of FR 900482", in: J. Am. Chem. Soc. , 1993 , 115 , pp. 6094-6100; doi: 10.1021 / ja00067a026 .
- ↑ Serge L. Beaucage, Radhakrishman P. Iyer: “Advances in the Synthesis of Oligonucleotides by the Phosphoramidite Approach”, in: Tetrahedron , 1992 , 48 , pp. 2223-2311; doi: 10.1016 / S0040-4020 (01) 88752-4 .
- ↑ Michael Schelhaas, Herbert Waldmann: “Protection group strategies in organic synthesis”, in: Angewandte Chemie , 1996 , 103 , p. 2194; doi: 10.1002 / anie.19961081805 .
- ↑ Marco Eissen, Radoslaw Mazur, Heinz-Georg Quebbemann and Karl-Heinz Pennemann: "Atom Economy and Yield of Synthesis Sequences", in: Helv. Chim. Acta , 2004 , 87 , pp. 524-535; doi: 10.1002 / hlca.200490050 .
- ^ Marco Eissen, Jürgen O. Metzger: "Environmental Performance Metrics for Daily Use in Synthetic Chemistry", in: Chemistry - A European Journal , 2002 , 8 , pp. 3580-3585; doi: 10.1002 / chin.200247273 .
- ↑ Marco Eissen, Jürgen O. Metzger, Eberhard Schmidt, Uwe Schneidewind: 10 Years After “Rio” - Concepts for the Contribution of Chemistry to Sustainable Development , in: Angewandte Chemie , 2002 , 114 , pp. 402–425; doi : 10.1002 / 1521-3757 (20020201) 114: 3 <402 :: AID-ANGE402> 3.0.CO; 2-D .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , pp. 621–622.
- ↑ Hideaki Muratake, Harumi Kumagami, Mitsutaka Natsume: Synthetic studies of marine alkaloids hapalindoles. Part 3 Total synthesis of (±) -hapalindoles H and U , in: Tetrahedron , 1990 , 46 , pp. 6351-6360; doi: 10.1016 / S0040-4020 (01) 96007-7 .
- ↑ Hideaki Muratake, Mitsutaka Natsume: Synthetic studies of marine alkaloids hapalindoles. Part I Total synthesis of (±) -hapalindoles J and M , in: Tetrahedron , 1990 , 46 , pp. 6331-6342; doi: 10.1016 / S0040-4020 (01) 96005-3 .
- ↑ Hideaki Muratake, Mitsutaka Natsume: Synthetic studies of marine alkaloids hapalindoles. Part 2. Lithium aluminum hydride reduction of the electron-rich carbon-carbon double bond conjugated with the indole nucleus , in: Tetrahedron , 1990 , 46 , pp. 6343-6350; doi: 10.1016 / S0040-4020 (01) 96006-5 .
- ↑ Phil S. Baran, Thomas J. Maimone, Jeremy M. Richter: Total synthesis of marine natural products without using protecting groups , in: Nature , 446 , pp. 404-408; doi: 10.1038 / nature05569 .