Latrunculine
Latrunculin are macrolide - toxins by different marine produced living things. You are cytoskeletal inhibitors that reversibly actin - polymerization to microfilaments prevent. They are characterized by a thiazolidin- 2-one structure, which is rare for biomolecules, and have a toxic effect on a large number of animals , plants and cell lines .
Occurrence and history
They were discovered in 1983 in the sea sponge Negombata magnifica , which is native to the Red Sea and the Indian Ocean . The sponge Negombata magnifica from the class of horn silica sponges is about 70 cm tall, is reddish-brown in color and grows as a colony exposed and in large numbers. The sponges are noticeable because they rarely show feeding damage, although they are not hidden between corals and rocks . When touched, the sponge releases a reddish liquid from which fish swim away. If they cannot escape, as in an aquarium , the secretion is deadly. The poisoned fish show symptoms such as hemorrhage and loss of balance within seconds . In 1983, the toxins latrunculin A and B, later also C and M, the latrunculins G and H were isolated from this secretion with the addition of formaldehyde . Latrunculin S could later be isolated from Fasciospongia rimosa and Latrunculin T from Negombata magnifica. 1986 the first synthesis of latrunculin B, 1990, the synthesis of latrunculin A. In the following years it turned out that latrunculin also in various sea snails of the family of magnificent slugs and other sponges as Spongia mycofijiensis occur.
properties
Latrunculins are waxy solids and are typically dissolved in DMSO or ethanol . Both latrunculins are poorly soluble in water and in solution, latrunculin A is more stable than latrunculin B. However, in the presence of acids or alkalis, latrunculin A breaks down faster than latrunculin B.
| Latrunculine | ||||||||||
| Surname | Latrunculin A | Latrunculin B | Latrunculin C | Latrunculin D | Latrunculin G. | Latrunculin H. | Latrunculin M. | Latrunculin S. | Latrunculin T | |
| other names | (4 R ) - [(1 R , 4 Z , 8 E , 10 Z , 12 S , 15 R , 17 R ) -17-hydroxy-5,12-dimethyl-3-oxo-2,16-dioxabicyclo [13.3 .1] nonadeca-4,8,10-trien-17-yl] thiazolidin-2-one | Latrunculin C-15 ketone, 11 methyl ether | N - (hydroxymethyl) latrunculin A | N - (hydroxymethyl) latrunculin B | ||||||
| Structural formula |
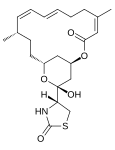
|

|

|

|

|

|
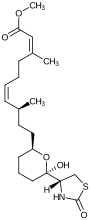
|

|
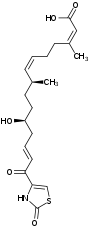
|
|
| CAS number | 76343-93-6 | 76343-94-7 | 76376-32-4 | 98155-17-0 | 122876-69-1 | 122876-74-8 | 122876-49-7 | 175992-99-1 | 877381-96-9 | |
| PubChem | 445420 | 6436219 | 44589658 | 6442448 | 10093792 | 15939613 | ||||
| Molecular formula | C 22 H 31 NO 5 S | C 20 H 29 NO 5 S | C 20 H 31 NO 5 S | C 21 H 31 NO 5 S | C 23 H 33 NO 6 S | C 21 H 31 NO 6 S | C 21 H 33 NO 5 S | C 22 H 33 NO 5 S | C 20 H 27 NO 5 S | |
| Molar mass | 421.55 g mol −1 | 395.51 g mol −1 | 397.53 g mol −1 | 409.54 g mol −1 | 451.58 g mol −1 | 425.54 g mol −1 | 411.56 g mol −1 | 423.57 g mol −1 | 393.50 g mol −1 | |
| Physical state | firmly | firmly | ||||||||
| Brief description | waxy solid | yellow solid | ||||||||
| Melting point | ||||||||||
| solubility | soluble in DMSO and ethanol | soluble in ethanol | ||||||||
|
GHS labeling |
|
|
||||||||
| H and P phrases | no H-phrases | no H-phrases | ||||||||
| no P-phrases | no P-phrases | |||||||||
| Wikidata | Q4255014 | Q4255012 | ||||||||
effect
Latrunculin-A and -B prevent the binding of monomeric G-actin to the nucleotide adenosine triphosphate (ATP). Only G-actin bound to ATP can polymerize to filamentous F-actin and so latriculins disrupt the structure of the cytoskeleton . Latrunculins have a very specific effect: in contrast to many cytoskeleton inhibitors, they only interact with actin and leave microtubules intact. Latrunculin A is more potent than latrunculin B, with both being over 100 times more potent than cytochalasin .
use
Latrunculins have no medicinal uses, but are produced for basic research. They are used for investigations on the cytoskeleton, were partly responsible for the discovery of cadherin and change the electrical activity of nerve cells . Latrunculin derivatives have potential as novel chemotherapeutic agents .
See also
The phallotoxins of the green and the white death cap fungus bind irreversibly to F-actin and hinder the depolymerization of the microfilaments. The colchicine of the autumn crocus , the chemotherapeutic agent vinblastine , and paclitaxel , one of the toxins of the Pacific yew tree , have a particular effect on microtubules .
Individual evidence
- ↑ I. Spector, NR Shochet, D. Blasberger, Y. Kashman: Latrunculins-novel marine macrolides did disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. In: Cell motility and the cytoskeleton. Volume 13, Number 3, 1989, pp. 127-144, doi: 10.1002 / cm.970130302 , PMID 2776221 .
- ^ A b M. Coué, SL Brenner, I. Spector, ED Korn: Inhibition of actin polymerization by latrunculin A. In: FEBS Letters . Volume 213, Number 2, March 1987, pp. 316-318, PMID 3556584 .
- ^ A b I. Spector, NR Shochet, Y. Kashman, A. Groweiss: Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. In: Science . Volume 219, Number 4584, February 1983, pp. 493-495, PMID 6681676 .
- ^ Entry on Latrunculine. In: Römpp Online . Georg Thieme Verlag, accessed on August 31, 2015.
- ↑ R. Zibuck, NJ Liverton, AB Smith: Total synthesis of (+) - latrunculin B. In: Journal of the American Chemical Society . Volume 108, Number 9, April 1986, pp. 2451-2453, doi: 10.1021 / ja00269a056 , PMID 22175603 .
- ^ AB Smith, I. Noda, SW Remiszewski, NJ Liverton, R. Zibuck: Total synthesis of (+) - latrunculin A. In: The Journal of Organic Chemistry . Volume 55, Number 13, 1990 pp. 3977-3979, doi: 10.1021 / jo00300a006 .
- ↑ a b J.D. White and M. Kawasaki: Total synthesis of (+) - latrunculin A. In: Journal of the American Chemical Society . Volume 112, Number 12, 1990 p. 4991-4993, doi: 10.1021 / ja00168a071 .
- ^ Y. Kakou, P. Crews, GJ Bakus: Dendrolasin and Latrunculin A from the Fijian Sponge Spongia mycofijiensis and an Associated Nudibranch Chromodoris lochi. In: Journal of Natural Products . Volume 50, Number 3, 1987 p. 482-484, doi: 10.1021 / np50051a023 .
- ^ A b W. E. Houssen, M. Jaspars, KN Wease, RH Scott: Acute actions of marine toxin latrunculin A on the electrophysiological properties of cultured dorsal root ganglion neurones. In: Comparative Biochemistry and Physiology - Part C Toxicology & Pharmacology . Volume 142, Number 1-2, 2006 Jan-Feb, pp 19-29, doi: 10.1016 / j.cbpc.2005.09.006 , PMID 16280258 .
- ↑ Latrunculine data sheet at Thermofisher, accessed on August 23, 2015.
- ↑ Latrunculine data sheet at Tocris, accessed on 23 August 2015.
- ↑ Latrunculin A data sheet from Sigma-Aldrich , accessed on August 23, 2015 ( PDF ).
- ↑ a b c d data sheet Latrunculin B from Latruncula magnifica, ≥80% (HPLC), solid from Sigma-Aldrich , accessed on August 23, 2015 ( PDF ).
- ^ Ian W. Southon, John Buckingham: Dictionary of Alkaloids, Second Edition with CD-ROM . CRC Press, 1989, ISBN 978-0-412-24910-5 , pp. 631 ( limited preview in Google Book search).
- ^ John W. Blunt, Murray HG Munro: Dictionary of Marine Natural Products with CD-ROM . CRC Press, 2007, ISBN 978-0-8493-8217-8 , pp. 1271 ( limited preview in Google Book search).
- ↑ a b c Data sheet Latrunculin A, from sea sponge, ≥85% (HPLC), waxy solid from Sigma-Aldrich , accessed on August 28, 2015 ( PDF ).
- ↑ EG Yarmola, T. Somasundaram, TA Boring, I. Spector, MR Bubb: Actin-latrunculin A structure and function. Differential modulation of actin-binding protein function by latrunculin A. In: Journal of Biological Chemistry . Volume 275, Number 36, September 2000, pp. 28120-28127, doi: 10.1074 / jbc.M004253200 , PMID 10859320 .
- ↑ JJ Bounhiol, M. Delsol: [Influence of the environment on the metamorphosis of amphibians (temperature, light, molecular concentration of water)]. In: Problèmes actuels d'endocrinologie et de nutrition. Volume 19, 1975, pp. 265-278, PMID 1085932 .
- ↑ T. Wakatsuki, B. Schwab, NC Thompson, EL Elson: Effects of cytochalasin D and latrunculin B on mechanical properties of cells. In: Journal of cell science. Volume 114, Pt 5 March 2001, pp. 1025-1036, PMID 11181185 .
- ↑ MA Khanfar, DT Youssef, KA El Sayed: Semisynthetic latrunculin derivatives as inhibitors of metastatic breast cancer: biological evaluations, preliminary structure-activity relationship and molecular modeling studies. In: ChemMedChem . Volume 5, number 2, February 2010, pp. 274-285, doi: 10.1002 / cmdc.200900430 , PMID 20043312 , PMC 3529144 (free full text).
- ↑ H. Konishi, S. Kikuchi, T. Ochiai, H. Ikoma, T. Kubota, D. Ichikawa, H. Fujiwara, K. Okamoto, C. Sakakura, T. Sonoyama, Y. Kokuba, H. Sasaki, T. Matsui, E. Otsuji: Latrunculin a has a strong anticancer effect in a peritoneal dissemination model of human gastric cancer in mice. In: Anticancer Research . Volume 29, Number 6, June 2009, pp. 2091-2097 , PMID 19528469 .
