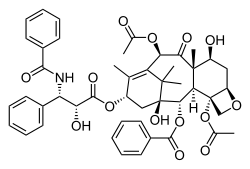
Paclitaxel
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Paclitaxel | |||||||||||||||||||||
| other names |
(2α, 4α, 5β, 7β, 10β, 13α) -4,10-bis (acetyloxy) -13 - {[(2 R , 3 S ) -3- (benzoylamino) -2-hydroxy-3-phenylpropanoyl] oxy } -1,7-dihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl-benzoate ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 47 H 51 NO 14 | |||||||||||||||||||||
| Brief description |
white solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 853.92 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
213-216 ° C |
|||||||||||||||||||||
| solubility |
Depending on the modification and the measuring method, solubilities of approx. 0.1 mg · l −1 up to 30 mg · l −1 in water are given. |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Paclitaxel ( Taxol ) is used as a medicine to treat various types of cancer (e.g. breast cancer ).
Occurrence
Paclitaxel is a substance from the group of taxanes that occurs in the bark of the Pacific yew tree ( Taxus brevifolia ) .
properties
Paclitaxel is a colorless solid at room temperature . In water it is barely soluble. There are at least three crystalline forms, one of which is a dihydrate . There are also glassy solidified modifications with a glass transition around 152 ° C described. The various information on solubility can be attributed to the presence of different crystal structures or the formation of a less soluble dihydrate in aqueous solution.
Extraction

Paclitaxel can be obtained from the Pacific yew tree ( Taxus brevifolia ). Since this type of yew tree is not very common and the taxol content is low, the world demand for paclitaxel cannot be met in this way. This original manufacturing process requires the bark of 12 felled trees for 1 gram of paclitaxel and a post-treatment time of around 9 weeks. For some years now, paclitaxel has been partially synthesized from baccatin III , which is found in the needles of the European yew tree ( Taxus baccata ). This is done using the Ojima-Holton method , which was developed by Iwao Ojima , among others . Another method for industrial production is the biotechnological extraction of paclitaxel from yew cell cultures.
A total synthesis of paclitaxel was first described in 1994 by Kyriacos C. Nicolaou , but found no industrial use. Another synthetic option using the Chan rearrangement was found in the same year by Robert A. Holton .
It has been demonstrated that paclitaxel by the endophytic living fungal Taxomyces andreanae biosynthesized which symbiotically lives in or on plants.
discovery
Monroe E. Wall and Mansukh C. Wani jointly conducted an extensive search for anti-cancer agents in the late 1960s. In 1971 they were able to isolate and characterize the substance paclitaxel by extracting the bark of the Pacific yew tree ( Taxus brevifolia ) and its anti-growth effect on cells, etc. a. Cancer cells .
Analytics
For a reliable qualitative and quantitative determination of paclitaxel, the coupling of HPLC with mass spectrometry can be used after adequate sample preparation . The method is suitable for detection in blood or plasma samples as well as in urine samples. Detection in dried blood samples is also possible with this analytical technique.
pharmacology
application areas
Paclitaxel is used in the therapy of malignant tumors ( chemotherapy ). Its areas of application include:
- Ovarian cancer (in combination with cisplatin or carboplatin ),
- Breast cancer (possibly in combination with trastuzumab )
- Non-small cell lung cancer (in combination with or carboplatin )
- Adenocarcinoma of the pancreas (in combination with gemcitabine )
- Prostate carcinoma (mainly the synthetic variant docetaxel ).
In addition to sirolimus , it is used in cardiac catheterization ( percutaneous transluminal coronary angioplasty , PTCA) to coat stents (“drug-eluting stents”) and drug-eluting balloon catheters (drug-eluting balloons), which reduces the risk of the coronary artery becoming blocked again should.
Mechanism of action
Paclitaxel works by preventing cells from dividing ( mitosis ); it thus belongs to the family of cytoskeleton inhibitors . It binds to β-tubulin and disrupts the breakdown of microtubules , which are part of the essential mitotic spindle in mitosis . In contrast to colchicine , vinblastine and nocodazole , which directly inhibit the formation of microtubules, paclitaxel inhibits their breakdown.
It therefore affects all dividing cells and can cause side effects. However, since cancer cells divide quickly compared to healthy cells, they are more affected.
Improved pharmacokinetics is achieved by formulating paclitaxel as paclitaxel albumin nanoparticles (see section nab-paclitaxel )
Side effects
During therapy with paclitaxel, the following side effects, which are characteristic of most cytostatic agents , were observed : bone marrow suppression with changes in the blood count ( thrombocytopenia , neutropenia , anemia ), neuropathies (especially paresthesia ), myalgia , hair loss , gastrointestinal side effects (e.g. nausea , vomiting) , Diarrhea ).
Pharmaceutical information
Paclitaxel is sparingly soluble in water and must be replaced by suitable solubilizers such as B. Ethanol and Macrogolglycerolricinoleate ( Cremophor ) for the therapeutically required concentrations in solution. Macrogol glycerol ricinoleate often leads to hypersensitivity reactions ( allergic reactions ). Alternative, macrogolglycerol ricinoleate-free dosage forms are nanoparticulate formulations such as paclitaxel bound to human albumin or encapsulated in liposomes .
In what is known as nab-paclitaxel ( n anoparticle a lbumin b ound paclitaxel ), paclitaxel is bound to albumin nanoparticles with an average size of approximately 130 nanometers. The powder formulated as a lyophilisate (trade name Abraxane , EU-wide approved in January 2008) is reconstituted with isotonic saline solution to an infusible suspension immediately before use . Due to the lack of solubilizers, no premedication against hypersensitivity reactions (HSR) is required. The nanoparticles themselves also cause the active ingredient to be better distributed in the body, which leads to linear pharmacokinetics . In contrast to conventionally formulated taxanes, higher dosages are possible.
The transport as a hydrophilic macromolecule in the bloodstream is responsible for an increased tumor-directed mechanism of action. The caveolar transport mechanism results in a selective exit from the bloodstream. Paclitaxel diffuses directly into the tumor cells and leads to apoptosis of the tumor cells. Overall, this leads to more active ingredient accumulation in the tumor and less in healthy tissue.
nab-Paclitaxel is - under the trade name Abraxane ® - approved in the EU in the indications of metastatic breast cancer (breast cancer), metastatic pancreatic cancer (pancreatic cancer) and non-small cell lung cancer (bronchial carcinoma) and is being further developed in various indications. a. also for skin cancer (melanoma) .
Liposomally encapsulated paclitaxel is used in China (trade name Lipusu ) for the treatment of ovarian cancer , as well as accompanying radiotherapy for breast cancer and in the treatment of inoperable non-small-cell lung cancer . In Europe, EndoTAG-1, a liposomal paclitaxel formulation for the treatment of pancreatic cancer, which was assigned the status of an orphan drug in 2006 , is being developed.
Trade names
Abraxane (EU), Celltaxel (D), Ebetaxel (A), NeoTaxan (D), Paxene (A), Ribotax (D), Taxol (D, A, CH), Taxomedac (D), other generics (D, A , CH)
See also
literature
- Eckhard Leistner: The biology of the taxanes. In: Pharmacy in our time . Volume 34, 2005, pp. 98-103. doi: 10.1002 / pauz.200400108
- Hans-Peter Lipp, Carsten Bokemeyer: Therapy of solid tumors: effectiveness and toxicity of the taxanes. In: Pharmacy in our time. Volume 34, 2005, pp. 128-137. doi: 10.1002 / pauz.200400113
- Volker Bartsch: The Taxol Book: 55 tables . 2., ext. and updated edition. Thieme, Stuttgart / New York 2004, ISBN 3-13-105462-X .
- William J. Gradishar, Sergei Tjulandin, Neville Davidson, Heather Shaw, Neil Desai, Paul Bhar, Michael Hawkins, Joyce O'Shaughnessy: Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared With Polyethylated Castor Oil-Based Paclitaxel in Women With Breast Cancer . In: Journal of Clinical Oncology . tape 23 , no. 31 , November 1, 2005, pp. 7794-7803 , doi : 10.1200 / JCO.2005.04.937 ( ascopubs.org ).
- William J. Gradishar, Dimitry Krasnojon, Sergey Cheporov, Anatoly N. Makhson, Georgiy M. Manikhas, Alicia Clawson, Paul Bhar: Significantly Longer Progression-Free Survival With nab-Paclitaxel Compared With Docetaxel As First-Line Therapy for Metastatic Breast Cancer . In: Journal of Clinical Oncology . tape 27 , no. 22 , August 1, 2009, p. 3611-3619 , doi : 10.1200 / JCO.2008.18.5397 ( ascopubs.org ).
Web links
- More about Taxol
- Biosynthesis of Paclitaxel
- Scientific literature in Pubmed
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Paclitaxel
Individual evidence
- ↑ Paclitaxel data sheet from Acros, accessed on February 20, 2010.
- ↑ Data sheet Paclitaxel, Semi-Synthetic (PDF) from Calbiochem, accessed on December 7, 2015.
- ^ Liggins et al: Solid-state characterization of paclitaxel. In: J. Pham. Sci. Volume 86, 1997, pp. 1458-1463. PMID 9423162 .
- ↑ a b Data sheet Paclitaxel from Taxus brevifolia, ≥95% (HPLC) from Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ^ Entry on paclitaxel in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ KV Rao: Taxol and related taxanes. I. Taxanes of Taxus brevifolia bark . In: Pharmaceutical Research . tape 10 , no. 4 , April 1, 1993, pp. 521-524 , PMID 8097872 .
- ↑ Paclitaxel from fermenters. In: Pharmaceutical newspaper . Edition 34/2002, (online) .
- ↑ MC Wani, HL Taylor, ME Wall, P. Coggon, AT McPhail: Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. In: J. Am. Chem. Soc. Volume 93, No. 9, 1971, pp. 2325-2327, doi: 10.1021 / ja00738a045 .
- ↑ S. Malhi, N. Stesco, S. Alrushaid, TM Lakowski, NM Davies & X. Gu: Simultaneous quantification of reparixin and paclitaxel in plasma and urine using ultra performance liquid chromatography-tandem mass spectroscopy (UHPLC-MS / MS): Application to a preclinical pharmacokinetic study in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 1046, Mar 1, 2017, pp. 165–171. PMID 28187377
- ↑ F. Xie, E. De Thaye, A. Vermeulen, J. Van Bocxlaer & P. Colin: A dried blood spot assay for paclitaxel and its metabolites. J Pharm Biomed Anal. 148, Jan 30, 2018, pp. 307-315. PMID 29078175
- ↑ a b c Technical information Abraxane . July 2015.
- ↑ Summary of the product characteristics , European Medicines Agency, EMA (German) .
- ↑ Summary of the European Public Assessment Report (EPAR) European Medicines Agency, EMA (English) .
- ↑ a b Summary of the EPAR for the public (PDF; 116 kB) European Medicines Agency, EMA (German) .
- ↑ VTG Chuang u. a .: Pharmaceutical Strategies Utilizing Recombinant Human Serum Albumin. In: Pharmaceutical Research. Volume 19, Number 5, 2002, pp. 569-577. doi: 10.1023 / A: 1015396825274 .
- ↑ N. Desai et al. a .: Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. In: Clin Cancer Res . 12, 2006. doi: 10.1158 / 1078-0432.CCR-05-1634
- ↑ ABRAXANE ® Demonstrates Significant Improvement in Progression-Free Survival Compared to Standard Chemotherapy in Advanced Melanoma Patients Press release on melanoma from Celgene International Sàrl (English) .
- ↑ Orphan designation EU / 3/06/419
- ↑ Red List Online, as of August 2009.
- ↑ Swiss Medicines Compendium , as of August 2009.
- ↑ AGES-PharmMed, as of August 2009.

