Bronchial carcinoma
| Classification according to ICD-10 | |
|---|---|
| C34 | Malignant new growth of the bronchi and lungs |
| C34.0 | Main bronchus, Carina tracheae, Hilus |
| C34.1 | Upper lobe |
| C34.2 | Middle lobe |
| C34.3 | Lower lobe |
| C34.8 | Bronchus and lungs, overlapping several areas |
| C34.9 | Localization unspecified |
| ICD-10 online (WHO version 2019) | |

Under a lung cancer (including lung carcinoma , bronchogenic carcinoma , bronchial carcinoma , lung cancer ; English bronchial carcinoma , lung cancer ) refers to a malignant neoplasm degenerate cells of the bronchi and bronchioles . Lung carcinoma is one of the most common malignant diseases in humans.
The inhalation of tobacco smoke is by far the most important risk factor for lung cancer. The risk increases with the amount and duration of smoking. In men it is responsible for the development of bronchial carcinoma in around 90% and in women in around 80% of cases. The second most common cause is living in rooms heavily contaminated with the radioactive noble gas radon . There are also numerous other substances that can trigger the tumor (for example asbestos or chromium ), to which one can be exposed for occupational or environmental reasons.
The healing rate depends to a large extent on the type of cancer and its extent. She is z. B. in the case of small cell lung cancer is still very bad and has a five-year survival rate of less than ten percent.
More people die from lung cancer than from breast cancer , prostate cancer and colon cancer combined. If the patient develops the first symptoms (e.g. chronic hoarseness or coughing up blood), it is usually too late for successful therapy. But if lung cancer is discovered early (mostly by chance), the American Cancer Society (ACS) has a survival rate of 47%. At the same time, the x-ray of the lungs is the most frequently performed x-ray examination with a 50% share. The accidental detection of lung cancer in the early stages (stage 1) in the X-ray image is difficult, however. It is known that round foci of five to ten millimeters can easily be overlooked.
Epidemiology

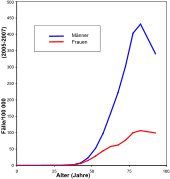
25 percent of all malignant tumors (malignancies) are bronchial carcinomas. It is the most common type of cancer in men worldwide; in Germany the third most common after prostate and colorectal carcinoma . It ranks first among men and second among women as the cause of cancer deaths. The incidence in Central Europe is around 60 per 100,000 inhabitants. The number of new cases - in Germany around 50,000 per year - shows an upward trend. Among the causes of death in Germany, it ranks fourth with around 40,000 deaths a year, and even third among men. The age peak of the diseases is around the age of 60. The average five-year survival rate (i.e. how many people are still alive after five years) is 15 percent for men and 20 percent for women. The life expectancy of the individual patient is very much dependent on the stage of the disease ( TNM classification ) and the subtype (see below).
An increasing incidence in women is observed; Lung cancer will soon also be the leading cause of cancer death in women in Europe, and this is already the case in Great Britain and Poland. The ratio of sick men to women is around 3: 1, whereby the changes in tobacco consumption behavior of women are increasingly converging (see diagram). Environmental influences or genetic causes can be significant; the prognosis is somewhat better for women. Non-smoking lung cancer is more common in women than in men. In the United States , too , the lung cancer rate was always higher in men than in women. However, a recent survey shows that the incidence of lung cancer in women among young white Americans is higher than that in men. This cannot be adequately explained by smoking behavior alone.
causes
The main cause of tumor development is tobacco smoke . It contains about 2000 substances, of which at least 100 are carcinogenic ( e.g. tar and a large number of other hydrocarbon compounds ). It has been shown that the benzo [ a ] pyrene contained in tobacco smoke can damage the p53 protein known as a tumor suppressor . 95 percent of all patients with lung cancer are smokers or former smokers. 30 to 40 years after starting to smoke, the risk of illness in heavy smokers is up to 60 times higher than in non-smokers. Passive smokers also breathe in the same substances as smokers, albeit in much lower concentrations. The risk for non-smokers who are permanently exposed to passive smoke is therefore only moderately increased with 1.2 to 1.3 times that of non-exposed non-smokers. The so-called British Doctors Study demonstrated the connection between lung cancer and active smoking as early as the 1950s. The likelihood of developing lung cancer by the age of 75 is 1 in 6 for men who have smoked throughout their adult lives. For men who have given up smoking by the age of 60, 50, 40 and 30, the probability is 1:10, 1:16, 1:33 and 1:50. For men who have never smoked, the probability is only about 1 in 250. While bronchial carcinoma was still a “very rare tumor” in 1913, with 337 cases described in the entire literature , in 2008 there were around 1.38 million deaths worldwide because of it. In countries like the USA, where the proportion of smokers has been falling again since the 1960s, there is now also a clear decline in lung cancer, which, with a time lag of 20-30 years, runs almost parallel to the decline in tobacco consumption.
Genetic studies of lung tissue have shown that the activity of genes that are responsible for repairing DNA and which are supposed to stop the development of lung cancer is permanently reduced, even in ex-smokers.
The second most common cause of lung cancer is the radioactive gas radon . In Germany there are an estimated 1900 deaths from lung cancer due to exposure to radon. Its radon decay products, which are also radioactive, are deposited in the bronchial tubes during inhalation and accumulate there. They irradiate the tissue with biologically very effective alpha particles . In some areas of Germany, including in eastern Bavaria, Saxony and Thuringia as well as in Breisgau, the soil materials in poorly ventilated cellars can lead to increased radon levels. The World Health Organization ( WHO) has set a target value of 100 Becquerel per cubic meter of indoor air. In Switzerland, radon is the cause of around ten percent of all bronchial carcinoma cases. Lung cancer is a recognized occupational disease among uranium miners.
Other toxins like asbestos , uranium , chromium compounds, mustard gas , polycyclic aromatic hydrocarbons and nickel are also considered carcinogenic. This increases the risk of illness many times over if the patient is also a smoker. For example, exposure to asbestos increases the risk of illness for non-smokers by five times and for smokers by ninety times. A familial accumulation suggests a genetic component. Chronic inflammatory irritation also has an influence on the development of bronchial carcinoma. They can also develop in the area of old lung scars, as they occur after tuberculosis , silicosis or pulmonary infarction .
How diet and lung cancer, i.e. bronchial carcinoma, could be related has been recorded in the EPIC study since 1992. In the study, more than 500,000 initially healthy participants from ten European countries are recorded with regard to their eating behavior. In addition, the weight, height and body fat distribution of the participants are recorded and blood tests are carried out. Since around 2000, all new cancer cases and other chronic diseases have been recorded and associated with eating habits and lifestyle . Over the years, more and more knowledge has been gained about the composition of a “healthy” diet , which could offer potential protection against cancer and other diseases. In April 2007, scientists from the study centers at the German Cancer Research Center (DKFZ) in Heidelberg and at the German Institute for Nutritional Research (DIFE) in Potsdam -Rehbrücke took stock: A high consumption of fruit and vegetables protects against lung cancer and cancer of the upper digestive tract . Men who consume less benefit in particular from an increase to 300 g of fruit and vegetables per day.
Emergence
The development of bronchial carcinoma is very complex and has not yet been fully deciphered. A simplification of the most common development model: the reserve cells of the bronchial system are pluripotent , i.e. they are able to differentiate into different cells of the bronchial mucosa, for example squamous epithelia , goblet cells, club cells (mucus-producing cells of the bronchioles) and neuroendocrine cells. After chronic inflammatory irritation and exposure to carcinogenic noxae , the epithelium can react with squamous cell metaplasia, goblet cell hyperplasia or degeneration of neuroendocrine cells that form the various subtypes of bronchial carcinoma.
Symptoms
The dangerous thing about all lung tumors is that they only become clearly noticeable late.
Symptoms such as cough, fever, chest pain and difficulty breathing ( dyspnea ) appear first ; in heavy smokers these are rarely new or unusual findings. Paralysis of the respiratory muscles due to phrenic nerve palsy (the phrenic nerve controls the movement of the diaphragm), hoarseness due to recurrent palsy (the recurrent laryngeal nerve wraps around the aorta and leads back to the larynx muscles), Horner's syndrome and Pancoast's syndrome when the cancer cells develop under destruction of tissue affects nerves, muscles and bones.
One by exudate incurred pleural effusion and a paraneoplastic syndrome need a lot of tumor cells to become conspicuous. A tumor also has to be several centimeters in size for the influence of congestion, that is, the backflow of venous blood in front of the heart. Not every type of lung cancer causes these symptoms. Especially with small cells, metastases in the brain, bones, heart and other organs can cause the first symptoms.
Pathology of the subtypes
Histological classification of lung cancer according to the World Health Organization (WHO)
- Small cell lung cancer ( SCLC = small cell lung carcinoma ): oat cell carcinoma ( oat cell carcinoma ) Intermediary type, Combined oat-cell-type (about 15%)
-
Non small cell lung cancer ( NSCLC = non small cell lung carcinoma , 85%)
- Squamous cell carcinoma: spindle cell
- Adenocarcinoma: acinar, papillary, bronchiolo-alveolar, solid with mucus formation
- large cell carcinoma: giant cell carcinoma, clear cell carcinoma
- Other types of carcinoma: adenosquamous carcinoma, sarcomatoid carcinoma, neuroendocrine carcinoma (NEC)
The distinction between SCLC and NSCLC is based on differences in biological behavior, prognosis, and treatment options.
TNM classification
This classification applies to both small cell ( SCLC ) and non-small cell carcinoma ( NSCLC ) as well as bronchopulmonary carcinoids . Sarcomas and other rare tumors are not included .
| TNM | criteria | ||
|---|---|---|---|
| T | TX | Primary tumor cannot be assessed or Detection of malignant cells in sputum (coughed up) or in bronchial irrigation (1) |
|
| T0 | No evidence of a primary tumor | ||
| Tis | Carcinoma in situ (surface carcinoma without breaching the basement membrane) | ||
| T1 | Tumor up to 3 cm in size | ||
| T1mi | Minimally invasive adenocarcinoma | ||
| T1a | Tumor up to 1 cm in size | ||
| T1b | Tumor larger than 1 cm to 2 cm in size | ||
| T1c | Tumor over 2 cm to 3 cm in size | ||
| T2 | Tumor over 3 cm to 5 cm in size or Tumor affects the main bronchus (2) and is located at least 2 cm from the carina (bronchial bifurcation) or |
||
| T2a | Tumor over 3 cm to 4 cm in size | ||
| T2b | Tumor over 4 cm to 5 cm in size | ||
| T3 | Tumor over 5 cm to 7 cm in size or direct infiltration of the parital pleura (outer pleura) or |
||
| T4 | Tumor infiltrated: diaphragm or Mediastinum or |
||
| N | Nx | No statement can be made on regional lymph node metastases . | |
| N0 | No metastases in the regional lymph nodes. | ||
| N1 | Metastases in the ipsilateral peritoneal or ipsilateral hilar or intrapulmonary lymph nodes (4) | ||
| N2 | Metastases in the ipsilateral mediastinal or subcarinal lymph nodes (5) | ||
| N3 | Metastases in the contralateral mediastinal lymph nodes or contralateral hilar
lymph nodes or |
||
| M. | |||
| M0 | No distant metastases detectable. | ||
| M1 | The tumor has formed distant metastases. | ||
| M1a | Further tumor foci in a contralateral lung lobe or Tumor with pleural metastases or |
||
| M1b | Extra thoracic metastasis in an organ (outside of the chest) | ||
| M1c | Multiple extrathoracic metastases in one or more organs | ||
Notes: (1) T0: Sometimes it is not possible to detect a correct primary tumor. When examining coughed up secretion (sputum) or during a bronchoscopy with rinsing, tumor cells can be clearly detected.
(2) T2: The main bronchus is the large bronchus after the bifurcation (karina) of the windpipe.
(3) Lung tumors can block airways as they grow. The lungs behind it then collapse (atelectasis). If the bronchus is not completely blocked, a build-up of secretion leads to inflammation, which can affect an entire lung or parts of it. The picture is similar to pneumonia. Therefore, a lung tumor must be ruled out for any pneumonia that does not heal within 4 weeks.
(4) N1: Lymph nodes near the bronchi (= peribronchial) on the same side (= ipsilateral) or the equilateral hilus (lung root) or lymph nodes in the lung tissue (= intrapulmonary).
(5) N2: The mediastinum is the space between the lungs. Ipsilateral mediastinal lymph nodes are on the same side as the primary tumor. Subcarinal lymph nodes are located below the bifurcation of the bronchus.
(6) N3: Contralateral means on the opposite side of the tumor. Mediastinal lymph nodes lie between the two lungs, the hilar lymph nodes lie in the root of the lung. Scalenus lymph nodes lie next to the scalene muscles . Supraclavicular lymph nodes are located in the collarbone fossa .
(7) M1a: after the tumor has invaded the lung cavity or pericardial cavity, an effusion of liquid often forms in which tumor cells are usually detectable.
Small cell lung cancer (SCLC)
This also oat cell carcinoma or oat cell carcinoma , in the hospital jargon Klein Zeller called tumor accounts for 20 to 25 percent of lung cancer from. It originates from the neuroendocrine APUD cells (cells of the Kultschitzky type named after the histologist Nicholas Kultschitzky ), is mostly located in the center of the lungs and classically surrounds the larger bronchi like a cuff. Early lymphogenic (via lymphatic drainage pathways) and hematogenic (via blood vessels) metastasis usually means that metastases have formed before it is discovered (preferably in the brain , bones , liver and adrenal cortex ). An existing Lambert-Eaton-Rooke syndrome can be the first indication of the tumor. Due to their neuroendocrine origin, the cells of small cell lung cancer can produce hormones that are not normally produced in the lungs ( paraneoplasia ) and can affect the whole body (for example, the production of ACTH leads to Cushing's syndrome or antidiuretic hormone (ADH) the syndrome of inappropriate antidiuretic hormone secretion ). Small cell lung cancer has a very poor prognosis and is practically inoperable; chemotherapy is the palliative treatment of choice . Small cell lung cancer is usually not classified according to the TNM classification. Instead, a distinction is made between extensive and limited disease (only limited to the thorax) and very limited disease (only limited to one half of the thorax).
Histologically one can see densely packed, mostly fragile (squeeze artifacts!) Small tumor cells with hyperchromatic (dark blue) cell nuclei that flatten each other, with fine-dusted chromatin (salt-and-pepper chromatin) and inconspicuous / missing nucleoli. Neuroendocrine markers ( Chromogranin A , Synaptophysin, CD56 ) and an extremely high growth rate ( Ki-67 ) can often be detected immunohistochemically in SCLCs . With regard to CK18 , one typically finds a perinuclear punctiform reactivity. CK7 can be positive. Squamous markers like CK5 / 6 and p63 are mostly negative.
Squamous cell carcinoma
The squamous cell carcinoma is approximately 40 to 45 percent of the most common non-small Bronchialtumor. After chronic irritation of the mucous membrane, it arises from squamous cell metaplasia from squamous cell dysplasia. It is often localized at the branches of the subsegment bronchi. Its slow growth and early metastasis into the regional lymph nodes are characteristic . Macroscopically, ulcerations, necrosis, bleeding and pseudocystic transformation are common in this gray-white tumor. Histologically, a distinction is made between a keratinizing type and a non- keratinizing type . The latter has a poorer prognosis due to its lower differentiation. The five-year survival rate is between 20 and 1% depending on the stage (TNM; AJCC).
Histologically, in well-differentiated tumors one can see layered tumor cell aggregates reminiscent of squamous epithelium, made up of medium-sized to large tumor cells with broad eosinophilic cytoplasm, rather light cell nuclei with large nucleoli and possibly characteristic intercellular bridges and signs of cornification with the formation of "horn beads". Dysplasia through to carcinoma in situ in the bronchus epithelium suggest a lesion that has developed in the lungs itself. Otherwise (especially if there are no risk factors such as smoking, exposure to asbestos, Zn radio / chemotherapy or chronic lung diseases), metastases from squamous cell carcinomas in other locations, e.g. B. from the ENT tract, the skin or the cervix uteri may be considered. Glandular structures and mucus formation should not be visible or suggest an adenocarcinoma. Poorly differentiated tumors can be difficult to classify. Immunohistochemistry can help here. Squamous cell carcinomas are mostly positive for squamous cell markers such as CK5 / 6 and p63 and negative for CK7. TTF1 is rarely detectable. The growth rate (Ki-67) is significantly increased, but not as strong as in the SCLC.
Adenocarcinoma
Up to 20 percent of lung cancers are adenocarcinomas . They arise from mucus-producing cells and develop preferentially in scar tissue (e.g. old tuberculosis). The tumor is mostly in the peripheral lungs . Here, too, an early metastasis is typical, which takes place via the lymphatic system as well as via the bloodstream, preferably to the brain , liver and adrenal cortex . Histologically , one sees mainly glandular formations. The comparatively rare bronchioloalveolar carcinoma plays a special role . This special form, which makes up about one to nine percent of the bronchial carcinomas examined, grows slowly and within the anatomical structures. Radiologically it is therefore often mistaken for pneumonia . In contrast to other bronchial carcinomas, there is apparently no causal relationship to inhalative noxae.
Depending on the subtype, glandular, papillary or solid atypical epithelial structures with signs of mucus formation should be seen histologically. Immunohistochemically, adenocarcinomas are usually positive for CK7 and negative for squamous cell markers such as CK5 / 6 and p63. The growth rate (Ki-67) is significantly increased, but not as strong as in the SCLC. The detection of TTF1 suggests an adenocarcinoma developed in the lungs (DD metastasis of a thyroid carcinoma). If the presence of a metastasis or a malignant pleural mesothelioma infiltrating the lung is suspected , additional markers can be used to narrow down the localization of the primary tumor.
Large cell lung cancer
They make up 10 to 15 percent of bronchial carcinomas and are always a diagnosis of exclusion. They too metastasize hematogenously in the liver , brain and bones . Large cell bronchial carcinomas are probably dedifferentiated adeno- or squamous cell carcinomas that can no longer be recognized as such under a light microscope. Histologically, they show large cells with large nuclei, lots of cytoplasm and abundant nucleoli . Immunohistochemically and electron microscopically, however, they still show minimal signs of differentiation from glandular or squamous epithelium. A histological variant, like small cell lung cancer, is derived from neuroendocrine cells and shows rosette, trabecular or nest-like growth.
Others
The other types of carcinoma are rare and together make up less than ten percent of bronchial carcinomas. Often there are also mixed types. Metastases from other tumors from the cava flow area ( lung metastases ) are much more common .
Diagnosis
Imaging methods play an important role in the diagnosis of bronchial carcinoma . So these are X-ray of the thorax in two planes and computed tomography (CT) of the chest, the drug of choice for detecting and locating of lung cancer. The positron emission tomography (PET) can also be used for the detection of distant metastases addition. The bronchoscopy is for obtaining tissue samples for histological used assessment of the tumor. In laboratory diagnostics , the tumor markers neuron-specific enolase (NSE) and CYFRA 21-1 can also be used for diagnosis, but the results are not reliable.
In small cell lung cancer can also mediastinoscopy , the cranial computed tomography (CCT) or magnetic resonance imaging (MRI) of the head , an ultrasound ( "ultrasound") of the abdomen to rule out liver metastases , CT of the abdomen to rule out liver and adrenal metastases and a bone scan to exclude bone metastases .
The Liquid Biopsy can complement the diagnosis of tumors under certain conditions, for example when a primary tumor is found, in NSCLC. Here the liquid biopsy takes place to detect an EGFR - T790M mutation . Patients with EGFR sensitizing mutations treated with 1st or 2nd generation TKIs should be tested for a T790M mutation at the time of clinical or radiographic progression. The results should guide treatment decisions. Tests for the presence of EGFR-T790M as a resistance mechanism can be carried out either preferably from circulating tumor DNA (Circulating free DNA, cfDNA) from plasma using the liquid biopsy or from the DNA from the tissue using conventional biopsy.
If bronchial carcinoma is suspected, examinations are carried out to answer the following questions:
- What kind of tumor is it? How well is the tumor differentiated (grading)? (Species diagnosis)
- How far has the tumor progressed? (Spread diagnostics, staging , TNM classification )
- What are the options for operability in terms of maintaining lung function? (Determination of functional operability)
Despite the most modern imaging technology, the predictive power of computed tomography (CT) alone for assessing mediastinal lymph nodes is not sufficient for staging , so that mediastinoscopy or EBUS are of great importance here if suspicious lymph nodes are found in the CT. The extent to which positron emission tomography (PET) can replace mediastinoscopy is currently the goal of various studies. PET is of particular importance if, after induction therapy or prior surgery in the area of the mediastinum, tumor-free (negative) mediastinal lymph nodes decide on the indication for surgery, as mediastinoscopy in these cases has limited accuracy. The combination of the statements on the morphology and function of the lymph nodes by means of the fusion of CT and PET could enable further progress here.
- early detection
An earlier detection of bronchial tumors brings the possibility of an early and thus also effective therapy. A study published in 2011 was stopped early and as planned for this case after it had been shown before the planned end of the study that early detection through computed tomography screening reduced the risk of heavy smokers to die of lung cancer by 20 percent. The risk of overdiagnosis already mentioned in this study has now been confirmed in a new study. Up to 18 percent of lung tumors detected at an early stage would have no effect on quality of life or age, but their therapy would not cause negligible physical and psychological stress.
Small cell lung cancer (SCLC) therapy
Small cell lung cancer is usually inoperable at the time of diagnosis (70%). However, it often initially responds well to chemotherapy or radiation therapy (almost always palliative). However, very high doses of radiation must be applied in order to permanently destroy the tumor (tumor destruction dose 48–63 Gy). If the goal is curative, prophylactic cranial radiation should also be used to reduce cerebral recurrences. Thoracic irradiation> 40 Gy means there is a calculated risk of 1/20 that the patient will develop pulmonary fibrosis , which can sometimes be fatal. Other rare radiation sequelae are both myo- / pericarditis and myelitis , which can occur with a latency of nine months to one year. A burn of the esophagus with swallowing disorders is also possible. This means that life can be extended by a few months to a year. An operation is only possible in exceptional cases:
- If SCLC is found by chance during an operation to clarify a previously unclear pulmonary nodule that can only be diagnosed surgically (OP then according to the rules and with the radical nature of NSCLC).
- After extensive tumor destruction of a limited SCLC (limited disease) by chemotherapy to prevent the almost obligatory and then poorly treatable later relapse, as long as no lung removal is necessary.
-
Polychemotherapy
- ACO scheme ( adriamycin + cyclophosphamide + vincristine )
- CEV scheme ( carboplatin + etoposide + vincristine)
- PE scheme ( cisplatin + etoposide)
- Cisplatin + irinotecan
- Carboplatin + etoposide
- topotecan for relapse
-
Irradiation
- Pulmonary findings
- Skull (prophylactic or local in case of metastases )
Non-small cell lung cancer (NSCLC) therapy
The therapy options for the individual tumor stages are shown below. The presentation is based on the detailed guidelines of the American and European specialist societies. The therapy of NSCLC is subject to considerable changes due to increasing knowledge. The following overview therefore only gives an approximate impression of the therapeutic options.
Therapy with the EGFR tyrosine kinase inhibitor osimertinib resulted in a median progression-free survival of 18.9 months compared with 10.2 months compared to treatment with erlotinib or gefitinib . In November 2015, the US Food and Drug Administration (FDA) granted accelerated approval of osimertinib for the treatment of patients with advanced lung cancer with activating EGFR mutations. The European Commission followed suit with its approval. The drug has been approved in Germany since February 2016, and as a first-line therapeutic since June 7, 2018. The approval is based on the results of the FLAURA study. In addition to the EU, osimertinib is approved as a first-line therapeutic agent in the USA, Japan, Canada, Switzerland, Israel, Mexico, Australia, South Korea and other countries.
The staging corresponds to the TNM system (T: size of the tumor and its tissue infiltration : 0 = no tumor detectable, 1a = up to 2 cm, 1b => 2–3 cm, 2a => 3–5 cm, 2b => 5– 7 cm, 3 => 7 cm and / or additional involvement of other areas; N: lymph node involvement: 0 = no involvement, 1 = involvement of neighboring lymph nodes, 2 = involvement of distant lymph nodes, 3 = involvement of supraclavicular lymph nodes or lymph nodes on the other side of the lung; M: Metastasis: 0 = no metastases detectable, 1 = metastases detectable).
Occult non-small cell lung cancer
Occult non-small cell lung cancer is defined by the following clinical staging:
- TX, N1-3, M0
- TX, N0, M1
- TX, N1-3, M1
"Occult" is a bronchial carcinoma if the original tumor has not been found and the diagnosis is only z. B. was made by a cytological finding. The diagnosis is then mostly limited to a chest x-ray and a bronchoscopy. Short-term follow-up examinations (e.g. using CT) are necessary because the primary tumor can still show up in the course of the disease.
Tumors discovered in this way are usually still at an early stage. With timely surgery and complete surgical removal, these tumors can be cured. If the primary tumor can be found, further treatment depends on the exact tumor stage of the patient.
Patients with occult lung cancer are at increased risk of cancer elsewhere in the lungs, so regular follow-up care is required.
NSCLC stage 0
Stage 0 is described in the following staging group:
- Tis, N0, M0
NSCLC stage 0 corresponds to carcinoma in situ of the lungs. These tumors are defined by their non-invasive growth: they have not yet invaded the surrounding tissue and have not yet metastasized. Therefore, there is a good chance that such a tumor can be cured by surgical removal.
However, it is not uncommon for patients with in situ carcinoma to find additional, independent (primary) carcinomas in the lungs, many of which can no longer be operated on ( resectable ).
In a few patients considered suitable, endoscopic phototherapy with a hematoporphyrin derivative was suggested as a possible alternative to surgical removal. This treatment, which is currently in clinical trials, appears to be most effective for centrally located tumors that are discovered very early and extend less than an inch into the bronchus. Whether this form of treatment is really suitable for curing early-stage lung cancer has to be further researched.
Standard treatment:
Surgical removal (segment or wedge resection) with the aim of saving as much lung tissue as possible. Patients with carcinoma in situ of the lungs are at a very high risk of further lung tumors, so that further surgery may be necessary.
Endoscopic photodynamic therapy can be discussed as an experimental treatment .
NSCLC stage I.
Stage I non-small cell lung cancer has the following clinical stages:
- T1, N0, M0 → IA
- T2, N0, M0 → IB
Standard treatment
The usual treatment for stage I is complete surgical removal of the tumor. As before any operation, the patient's general medical condition must be carefully checked. The doctor must pay particular attention to the functional reserve of the lungs in order to assess whether the surgical treatment can be expected to benefit. The tumor focus can only be removed with the surrounding lung tissue, so that the vital capacity of the lungs is also reduced as a result of the operation . If the lung function is impaired before the operation, an operation can be difficult or impossible.
Treatment options:
- Lobectomy or segmental, wedge, or cuff resection as appropriate.
-
Radiation therapy with curative intention (for potentially resectable patients with medical contraindications for resection).
- “In the context of radiation therapy, there are new precision radiation techniques that enable precise delimitation of the tumor tissue. In this way, healthy tissue can be better protected. With such techniques, for example, so-called hypofractionated radiation is possible; that is, the necessary radiation dose is administered in a few fractions. Less consideration has to be given to the repairability of healthy tissue. The extreme form of precision radiation is so-called radiosurgery . Here, the tumor is usually given a high dose within a session of approx. 30–60 minutes. Innovative devices are developed with precision robotics, image location system and breathing compensation. Breathable tumors in particular, such as bronchial carcinoma, can move several centimeters when the patient breathes in and out. In order to continue to hit the target volume precisely, the external respiratory movement during radiosurgical treatment is recorded using a 3D camera and compared with the internal tumor position. For the patient, irradiation by means of radiosurgery therefore takes place without fixation or artificially induced respiratory arrest. Radiosurgery is non-invasive: no pain, no anesthesia, no surgery, but with results that are very comparable to surgery. "
- Clinical studies on adjuvant chemotherapy after resection.
- Studies on adjuvant chemoprevention.
- Endoscopic photodynamic therapy (currently in clinical testing; particularly suitable for selected patients in stage T1, N0, M0, no standard therapy)
Surgical risk
Immediate postoperative mortality depends on age, but a mortality of three to five percent after lobectomy (removal of a lung lobe) must be expected. For patients with impaired lung function, the doctor is more likely to suggest a segment or wedge resection of the tumor.
Exercise tests can help identify patients with impaired lung function who can still tolerate a lung resection. The availability of video-assisted thoracic wedge resection allows for limited resections in patients with impaired lung function who normally cannot perform a lobectomy.
Chances of recovery, risk of recurrence
Local recurrences (recurrence of the tumor at the site of the original tumor) were less likely in patients with stage I lung cancer who had received a lobectomy (removal of a whole lobe of the lung ) than in patients who had only a limited resection (segment or wedge resection) ) had been carried out. Regarding overall survival, however, no statistically significant differences could be determined. According to other scientific studies, there may be a survival benefit for lobectomy in patients with a tumor diameter greater than three centimeters, but not in tumors with a diameter of less than three centimeters. In each case, however, the rate of local recurrences after lobectomy was significantly lower, regardless of the size of the primary tumor.
Treatment options for inoperable patients
Patients with stage I inoperable disease who have sufficient lung reserves can be treated palliatively with radiation therapy.
In patients older than 70 years and suffering from an operable tumor (<4 cm), but who could not or did not want to be operated on for medical reasons, the five-year survival after radiation therapy was comparable to the surgical removal of the tumor.
In the two largest case series documented retrospectively (only after treatment was completed) on this problem, patients with inoperable disease who had received radiation therapy achieved five-year survival rates of 10 and 27 percent. Both case series showed that patients with T1, N0 tumors had better treatment results; in this subgroup, five-year survival rates of 60 and 32 percent were documented.
The operation complementary (adjuvant) therapy
Many patients who have been treated surgically nevertheless develop new tumors at the site of the original tumor or distant metastases. These patients might be better served with additional radiation or chemotherapy immediately after the operation.
A summary analysis ( meta-analysis )
of the scientific studies available so far on this topic compared postoperative radiation therapy with surgery alone. It showed a seven percent lower (!) Overall survival with adjuvant radiation therapy in patients with stage I or II bronchial carcinoma . As a qualification, it must be said about these data that it is unclear whether the results with modern radiation therapy might have been better. Radiation therapy technology has improved a lot in recent years. Today it is better than with older devices to focus the target volume of the radiation on the tumor and to keep the proportion of healthy tissue in the radiation field as small as possible.
NSCLC stage II
Stage II NSCLC is defined by the following clinical stage groups:
- T1, N1, M0
- T2, N1, M0
- T3, N0, M0
The treatment of choice for patients with stage II NSCLC is surgery. Before the operation, it is necessary to carefully check the patient's general medical condition. It is particularly important to estimate the functional reserve of the lungs. This depends on whether the surgical treatment can be of benefit. Immediate postoperative mortality (the risk of dying from complications of surgery) depends on age. One must reckon with a mortality of five to eight percent after pneumonectomy and three to five percent after lobectomy.
Patients with inoperable stage II and adequate lung reserves can be treated with radiation therapy and thereby also cured. Patients in excellent general condition can expect a three-year survival rate of up to 20 percent if the radiation therapy was carried out as planned and with the aim of achieving a cure. In the largest retrospective case series to date, 152 patients with NSCLC inoperable for medical reasons who had received definitive radiotherapy were documented. The five-year survival rate of these patients reached 10%; the patients with T1 tumors (44 of 152 patients) achieved disease-free survival of 60%.
Standard treatment options:
- Lobectomy; Pneumonectomy or segmental, wedge, or cuff resection depending on the surgeon's judgment
- Radiation therapy with curative intent (for potentially operable patients who have medical contraindications to surgery)
- Clinical studies on adjuvant chemotherapy with or without other types of treatment (chemotherapy, radiation therapy) after curative surgery
- Clinical studies on radiation therapy after complete removal of the visible tumor
NSCLC Stage IIIA
The NSCLC IIIA is defined by the following clinical stage groups:
- T1, N2, M0
- T2, N2, M0
- T3, N1, M0
- T3, N2, M0
Patients in clinical stage IIIA (N2) have a five-year survival rate of 10 to 15%. Patients with a large mediastinal tumor (= tumor visible on the X-ray ). in contrast, have a survival rate of two to five percent. Depending on the clinical circumstances, patients with stage IIIA non-small cell lung cancer (NSCLC) are treated with radiation therapy, chemotherapy, surgery, and combinations of these modalities. Although radiation therapy does not achieve a complete tumor response in most patients, there is a reproducible long-term survival benefit in five to ten percent of patients treated with standard therapy (fractionated radiation up to 60 Gy ). Often there is also good symptom control.
Patients in excellent general condition and those who only find out during the operation that the tumor cannot be surgically removed are very likely to benefit from radiation therapy.
The long-term results in patients with stage IIIA NSCLC are unfortunately still not favorable. Whenever possible, these patients should be treated in clinical studies in order to enable further progress.
The addition of modern chemotherapy based on cisplatin to radiation therapy can improve survival by up to ten percent compared with radiation therapy alone. The optimal sequence of chemotherapy and radiation therapy and the implementation of the chemotherapy administration has yet to be determined; it is currently being researched in clinical trials.
The results so far have been encouraging. Combined therapy with chemotherapy and surgery and / or radiation therapy should always be offered to patients in good general condition with NSCLC stage IIIA.
Standard treatment options
- sole operation in operable patients without a large tumor mass ("bulky disease")
- Radiation therapy alone in patients who cannot receive neoadjuvant chemotherapy plus radiation
- Chemotherapy in combination with other modalities
Special case: Pancoast / superior sulcus tumors (T3, N0 or N1, M0)
Tumors of the superior sulcus represent a separate group of diseases within bronchial carcinomas. They must be treated separately. Upper chest tumors are more invasive to grow in place and less prone to distant metastasis. Accordingly, a locally limited disease can still be curable, especially in the disease stage T3, N0. Radiation therapy alone, or followed by surgery, or surgery alone in selected cases, with five-year survival rates of 20 percent or more, can result in a cure for some patients. Patients with more invasive tumors in the area or true Pancoast tumors have a poorer prognosis and generally do not benefit from primary surgical therapy. However, an operation can be carried out later to document the complete response of the irradiated tumor and to remove the necrotic tissue. Simultaneous chemotherapy and radiation therapy followed by surgery can produce good results, especially in patients with stage T4, N0 or N1.
Standard treatment options:
- Radiation therapy and surgery
- radiation therapy alone
- sole operation (in selected cases)
- Chemotherapy in combination with other modalities
- Clinical studies with treatments with combined modalities
Special case: tumors of the chest wall (T3, N0 or N1, M0)
Patients with large primary tumors that directly infiltrate the chest wall can achieve long-term survival with surgery alone, provided the tumor has been completely resected.
Standard treatment options:
- Surgery.
- Surgery and radiation therapy.
- radiation therapy alone .
- Chemotherapy in combination with other modalities.
NSCLC stage IIIB
NSCLC IIIB includes the following clinical tumor stages:
- each T, N3, M0
- T4, each N, M0
Patients with stage IIIB NSCLC are not adequately treated with surgery alone. They are best treated with initial chemotherapy, chemotherapy plus radiation therapy, or radiation therapy alone. Treatment is determined depending on the exact location of the tumor and the general condition of the patient. Most patients in very good general condition are eligible for therapy with combined modalities. Patients with a malignant pleural effusion can rarely receive radiation therapy, but should be treated more like patients with stage IV tumor. Many randomized studies in patients with unresectable NSCLC stage III show that preoperative (neoadjuvant) or concurrent treatment with chemotherapy based on cisplatin and radiation of the chest are associated with an improvement in survival.
Patients with a tumor in stage IIIB are also eligible for thoracic irradiation in order to better control symptoms (cough, shortness of breath, hemoptysis, pain) if necessary.
- T4 or N3, M0
A patient with involvement of the supraclavicular lymph nodes who is otherwise well suited for curative radiation therapy is likely to survive three years. Although most of these patients do not fully respond to radiation therapy, this treatment often leads to an improvement in the tumor symptoms (palliation). Patients in very good general condition and those who only discover during surgery that the condition is inoperable will most likely benefit from radiation therapy. Some patients have a modest survival advantage with adjuvant chemotherapy. The addition of chemotherapy to radiation therapy has been associated with improvements in long-term survival in some prospective clinical trials, but not in all. A comprehensive analysis (meta-analysis) of the current state of knowledge showed an absolute survival advantage of four percent after two years if the radiation therapy was expanded to include chemotherapy based on cisplatin. The optimal sequence of the individual therapy modalities has yet to be determined and is currently being investigated in clinical studies.
Standard treatment options:
First line therapy
- radiation therapy alone
- Chemotherapy in combination with radiation therapy
- Chemotherapy and simultaneous radiation therapy
- platinum-based chemotherapy and therapy with bevacizumab (VEGF antibodies)
- chemotherapy alone
Second line therapy
- Erlotinib (EGFR inhibitor)
NSCLC stage IV
Stage IV of non-small cell lung cancer is defined by the following clinical stage:
- Every T, every N, M1
Patients with metastatic NSCLC respond subjectively and objectively to palliative chemotherapy based on cisplatin or carboplatin. Scientific studies have shown that in patients in stage IIIB or IV, cisplatin-based chemotherapy has a slight advantage in terms of short-term survival compared to the best possible supportive therapy. Although the toxic side effects can vary, the results of most studies based on platinum-containing combination therapy are similar. The therapy regimens that combine cisplatin with other substances show no significant differences in response, duration of response or survival. Patients in good general health with a limited number of distant metastases have better response and survival when receiving chemotherapy compared to other patients who receive supportive care only.
In a large-scale scientific study, the response rate for all patients was 19% and the median survival time was 7.9 months, regardless of the drug combination used, provided the treatment contained a platinum drug. Patients with a poor general condition experienced more toxic side effects and survived less than patients in good general condition.
According to the current state of knowledge, no special therapy protocol (based on a platinum drug) can be recommended as standard therapy. Outside of clinical studies, treatment should only be given to patients in good general condition with measurable / evaluable tumor lesions who, after having been fully informed about the expected risks and limited benefits, would like such a therapy.
Radiation therapy can be effective in providing targeted relief from symptoms of a localized infestation, such as: B. tracheal, esophageal or bronchial compression; Bone or brain metastases; Pain; Vocal cord paralysis; Hemoptysis or upper inflow congestion. In some cases, endobronchial laser and / or brachytherapy has been used to treat obstruction of the large airways (windpipe, bronchi). This therapeutic option can enable a patient in otherwise good general condition to continue an acceptable life.
In the rare case that a patient has a resectable lung tumor and a solitary brain metastasis at the same time, resection of the metastasis at the same time as the tumor is indicated. Appropriate post-operative chemotherapy and / or radiation therapy of the primary tumor site (and with post-operative whole-brain irradiation in daily fractions of 1.8-2.0 Gy ) is used to avoid the long-term toxic effects on normal brain tissue.
In asymptomatic patients that are closely observed, therapy can often be delayed until symptoms or signs of tumor progression appear.
Standard treatment options
- Radiation therapy , primarily with palliative intention in the case of local tumor growth
- Chemotherapy. The following therapy protocols are associated with similar survival rates:
- Cisplatin plus vinblastine plus mitomycin C
- Cisplatin plus vinorelbine
- Cisplatin plus paclitaxel
- Cisplatin plus docetaxel
- Cisplatin plus gemcitabine
- Carboplatin plus paclitaxel
- platinum-based chemotherapy and therapy with bevacizumab (VEGF antibodies)
- Therapy with osimertinib (3rd generation EGFR inhibitor)
The significantly better tolerated carboplatin (dosage according to AUC ) is probably not inferior to cisplatin in terms of its effect.
New substances for the therapy of advanced bronchial carcinoma
In recent years, new drugs for the treatment of lung cancer have become established. The basis for their development was and is the rapidly increasing knowledge of the biological basis of tumor development. These substances have a much more targeted effect than chemotherapy and are directed against certain surface features of the tumor. Since tumor development and metastasis is a very complex process, several different target structures have already been identified that are suitable as starting points for combating tumors. Some of the newly developed drugs have already proven themselves in large studies and have received approval for the treatment of bronchial carcinoma. They are bevacizumab , pemetrexed and erlotinib .
With Bevacizumab is a monoclonal antibody against vascular endothelial growth factor (VEGF, vascular endothelial growth factor is directed). The antibody intercepts this growth factor, which is essential for the tumor's blood flow, which means that no new blood vessels are created and existing vessels in and to the tumor also atrophy. This delays the progress of tumor growth. In particular in patients with adenocarcinoma-type tumor, a mean survival of more than 14 months could be achieved. Bevacizumab is used in addition to platinum-based chemotherapy for the first-line treatment of patients with unresectable advanced, metastatic, or recurrent NSCLC, except when the histology is predominant.
Erlotinib belongs to a group of tyrosine kinase inhibitors. These are small molecules that specifically inhibit the enzyme tyrosine kinase, which transmits signals that arrive via cellular receptors into the cell. Erlotinib specifically inhibits the transmission of growth signals via the tyrosine kinase of the EGF (Epidermal Growth Factor) receptor, which occurs in increased numbers on tumor cells and which is involved in the formation and growth of tumor cells. In this way, tyrosine kinase inhibitors can inhibit the growth of tumor cells. Erlotinib is approved in Germany for the second- and third-line therapy of advanced lung cancer in stage IIIB / IV, as the clinical studies showed a significant improvement in survival time and quality of life.
Due to its mechanism of action, pemetrexed is more like a modern chemotherapy drug.
A large number of other drugs for the targeted therapy of tumors such as bronchial carcinoma are in clinical trials. The status of these modern therapeutics cannot yet be conclusively assessed. In general, it is true of most drugs that they have considerably fewer side effects than the previously known chemotherapeutic agents. According to the data known so far, not all patients seem to benefit from targeted molecular therapy. As part of ongoing scientific study programs one tries to advance z. B. use a tissue sample to identify those patients who are likely to benefit most from the treatment.
Overview of the stages and therapies of NSCLC
| designation | Staging | Therapy options / standard treatments | Remarks |
|---|---|---|---|
| occult NSCLC | TX, N1-3, M0 TX, N0, M1 or TX, N1-3, M1 |
rather no operation, as it is conspicuous in distant metastasis stage IV, typically as CUP syndrome (cancer of unknown primary) | are usually not recognized at an early stage, as local or distant metastases are detected |
| NSCLC stage 0 | Tis, N0, M0 | Segment or wedge resection, possibly photodynamic therapy |
Carcinoma in situ |
| NSCLC stage I. | T1, N0, M0 = IA T2, N0, M0 = IB |
|
|
| NSCLC stage II | T1, N1, M0 T2, N1, M0 T3, N0, M0 |
|
|
| NSCLC Stage IIIA | T1, N2, M0 T2, N2, M0 T3, N1, M0 T3, N2, M0 |
|
so far no advantage for immunotherapy |
| Special case: Pancoast / superior sulcus tumors | T3, N0 or N1, M0 |
|
|
| Special case: tumors of the chest wall | T3, N0 or N1, M0 |
|
|
| NSCLC stage IIIB | each T, N3, M0 T4, each N, M0 |
|
|
| NSCLC stage IV | every T, every N, M1 |
|
Carboplatin better tolerated |
Use of chemotherapy in advanced stages
Benefits of palliative chemotherapy
Systemic treatment of NSCLC began in the 1970s, mainly with therapies containing doxorubicin and cyclophosphamide. Most of the time, the objective response of the tumor was short-lived and no influence on survival time was detectable. The discovery of cisplatin and its effectiveness in NSCLC led to its inclusion in various combination chemotherapies. While the response rates were higher than the older protocols without cisplatin, the toxicity was also higher. In addition, it was not possible to document a survival benefit in randomized studies during this time, which compared different chemotherapy regimens, so that the benefit of chemotherapy for this disease as a whole was called into question.
Chemotherapy compared to best possible supportive therapy
Several scientific studies have combination therapies or treatments to individual substances with the best supportive care ( best supportive care compared).
Not all studies showed a survival benefit for chemotherapy, and when it was demonstrable it was rather small but statistically significant. The frequent occurrence of treatment-related toxic side effects in many studies raised the question of whether the modest survival benefits could even be justified. Many studies have shown that the symptoms of lung cancer improved with therapy.
Optimal duration of palliative therapy
It is not clear how long the therapy should optimally be carried out in order to achieve the maximum survival benefit and the best possible control of the tumor symptoms. On the other hand, since chemotherapy is carried out with palliative intent, i. That is, in order to alleviate symptoms and, in the case of incurable disease, to extend the life span, a shorter treatment duration with less toxic side effects. This question has only been investigated in a few studies. Based on these studies and the guidelines for the management of non-resectable NSCLC of the American Society of Clinical Oncology, it is recommended that first-line therapy in patients with stage IV NSCLC be limited to four to six cycles.
literature
- V. Kumar, AK Abbas, N. Fausto (Eds.): Robbins and Cotrane Pathologic Basis of Disease. 7th edition. Elsevier Saunders, 2005, ISBN 0-7216-0187-1 , pp. 757-766.
- M. Classen, V. Diehl, K. Kochsiek: Internal medicine. 4th edition. Urban & Schwarzenberg, Munich 1998, ISBN 3-541-11674-9 , pp. 1459-1463.
- DP Berger, R. Engelhardt, R. Mertelsmann : The Red Book: Hematology and Internal Oncology. 3. Edition. Ecomed, Landsberg / Lech 2006, ISBN 3-609-51213-X .
- Stefan Fischer, Joachim Hartlapp, Wolfgang Wagner (eds.): Interdisciplinary treatment of lung cancer. Pabst Science Publ., Lengerich 2015, ISBN 978-3-95853-039-3 .
- Oncology guideline program (German Cancer Society, German Cancer Aid, AWMF): prevention, diagnosis, therapy and follow-up care for lung cancer. ( Long version 1.0, 2018, AWMF registration number 020 / 007OL ).
Web links
Guidelines / recommendations on diagnostics, therapy and follow-up care
- S3 guideline for the prevention, diagnosis, therapy and aftercare of lung cancer of the German Society for Pneumology and the German Cancer Society, AWMF -Register No. 020 / 007OL, status: 02/2018, valid until 12/2022
Current information, therapy studies, new drugs
- Cancernet, University Hospital Bonn: Basics » non-small cell ( memento from January 6, 2013 in the web archive archive.today ) and small cell ( memento from January 6, 2013 in the web archive archive.today ) bronchial carcinoma«
- National Cancer Institute (NCI )
Pathological recordings
- PathoPic - Image database of the University of Basel: Histological images of bronchial carcinomas
- PathoPic - Image database of the University of Basel: Macroscopic images of bronchial carcinomas
- PathoPic - Image database of the University of Basel: Cytological images of bronchial carcinomas
Epidemiology
Individual evidence
- ↑ American Cancer Society : Cancer Facts & Figures 2012. ( Memento from September 12, 2012 in the Internet Archive ) (PDF; 1.8 MB) Atlanta: American Cancer Society, 2012, p. 15.
- ^ National Cancer Institute: Tobacco Facts. Retrieved September 9, 2012.
- ↑ Anja Schröder: Radon is the second most common cause of lung cancer. Federal Office for Radiation Protection, press release from February 1, 2005 from the Science Information Service (idw-online.de), accessed on September 7, 2012.
- ↑ American Cancer Society ( Memento from June 12, 2006 in the Internet Archive ) (PDF)
- ↑ N. Wu et al .: Detection of small pulmonary nodules using direct digital radiography and picture archiving and communication systems. In: J Thorac Imaging. 21, 2006, pp. 27-31. PMID 16538152 .
- ↑ a b Cancer in Germany. RKI - Robert Koch Institute.
- ↑ Federal Statistical Office: Causes of Death 2013 .
- ↑ M. Malvezzi, P. Bertuccio, F. Levi, C. La Vecchia, E. Negri: European cancer mortality predictions for the year 2013. In: Ann Oncol . 2013. doi: 10.1093 / annonc / mdt010 .
- ↑ M. Serke: Gender-specific difference in lung cancer. In: Pneumology. 67 (5), May 2013, pp. 270-279.
- ↑ Ahmedin Jemal, Kimberly D. Miller, Jiemin Ma, Rebecca L. Siegel, Stacey A. Fedewa: Higher Lung Cancer Incidence in Young Women Than Young Men in the United States . In: New England Journal of Medicine . May 23, 2018, doi : 10.1056 / NEJMoa1715907 ( nejm.org [accessed December 13, 2018]).
- ↑ P. Vineis, N. Caporaso: Tobacco and cancer: epidemiology and the laboratory. In: Environ Health Perspect . 103, 1995, pp. 156-160. PMID 7737063 .
- ^ Richard Taylor, Farid Najafi, Annette Dobson: Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent . In: International Journal of Epidemiology . tape 36 , no. 5 , October 2007, ISSN 0300-5771 , p. 1048-1059 , doi : 10.1093 / ije / dym158 , PMID 17690135 .
- ^ German Cancer Research Center (ed.): Cancer diseases caused by smoking and passive smoking . Heidelberg, S. 4 ( dkfz.de [PDF]).
- ↑ Richard Peto, Sarah Darby, Harz Deo et al: Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. In: The BMJ . Volume 321, 2000, pp. 323-329 doi: 10.1136 / bmj.321.7257.323 .
- ^ J. R Siewert: Surgery. Edition 7. Gabler Wissenschaftsverlage, 2001, ISBN 3-540-67409-8 , p. 332. Limited preview in the Google book search
- ↑ J. Ferlay, HR Shin, F. Bray et al .: Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008 . In: International Journal of Cancer . tape 127 , no. December 12 , 2010, p. 2893-2917 , doi : 10.1002 / ijc.25516 , PMID 21351269 .
- ↑ A. Basters: The long breath of tobacco - cigarette smoke can switch genes on or off permanently. In: Image of Science. August 29, 2007.
- ↑ Radon causes lung cancer. In: bag.admin.ch . July 27, 2018, accessed September 19, 2019 .
- ↑ EPIC Potsdam study. ( Memento of March 27, 2014 in the Internet Archive ) Retrieved January 14, 2010.
- ↑ Adapted from: What protects against cancer and diabetes? In: MMW update. Med. 149. Jg., No. 24, 2007, p. 16. Quoted there from EPIC Symposium , Berlin, April 25, 2007.
- ↑ Berthold Jany, Tobias Welte: Pleural effusion in adults - causes, diagnosis and therapy. In: Deutsches Ärzteblatt. Volume 116, No. 21, 2019, pp. 377-385, here: pp. 379, 381 and 383 f.
- ↑ C. Wittekind (editor): TNM. Classification of malignant tumors. Eighth edition. Wiley-VCH Verlag 2017. ISBN 978-3-527-34280-8
- ^ Heinz-Peter Schmiedebach : Nicholas Kultschitzky. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 813.
- ↑ bronchial carcinoma. ( Memento from January 25, 2015 in the web archive archive.today ) University of Münster, accessed on March 21, 2012.
- ↑ D. Ayeni, K. Politi, SB Goldberg: Emerging Agents and New Mutations in EGFR-Mutant Lung Cancer. In: Clinical Cancer Research . Volume 21, number 17, September 2015, pp. 3818-3820, doi: 10.1158 / 1078-0432.CCR-15-1211 , PMID 26169963 , PMC 4720502 (free full text).
- ^ National Lung Screening Trial Research Team: Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. In: New England Journal of Medicine. 365, 2011, pp. 395-409, doi: 10.1056 / NEJMoa1102873 .
- ↑ EF Patz, P. Pinsky, C. Gatsonis, JD Sicks, BS Kramer, MC Tammemägi, C. Chiles, WC Black, DR Aberle: Overdiagnosis in Low-Dose Computed Tomography Screening for Lung Cancer. In: JAMA internal medicine. [electronic publication before printing] December 2013, ISSN 2168-6114 . doi: 10.1001 / jamainternmed.2013.12738 . PMID 24322569 .
- ↑ Tagrisso: Procedural steps taken and scientific information after the authorization , European Medicines Agency, accessed on February 28, 2019.
- ↑ AZD9291 Versus Gefitinib or Erlotinib in Patients With Locally Advanced or Metastatic Non-small Cell Lung Cancer (FLAURA) , ClinicalTrials.gov, US National Library of Medicine, last update January 24, 2019. Retrieved February 27, 2019.
- ↑ Jean-Charles Soria, Yuichiro Ohe u. a .: Osimertinib in Untreated-Mutated Advanced Non-Small-Cell Lung Cancer. In: The New England Journal of Medicine . 378, 2018, p. 113 ff, doi: 10.1056 / NEJMoa1713137 .
- ↑ TAGRISSO® (osimertinib) shows significant superiority over standard chemotherapy for non-small cell lung cancer with EGFR T790M mutation , oncotrends. Retrieved March 1, 2019.
- ↑ lungenkrebs.de
- ^ Arno Herzke: Stereotaxie - Radiosurgery - SBRT. Retrieved August 19, 2016 .