Bone metastasis
| Classification according to ICD-10 | |
|---|---|
| C79.5 | Secondary malignant neoplasm of bone and bone marrow |
| ICD-10 online (WHO version 2019) | |
Bone metastases , also called skeletal metastases or bony metastases , are malignant secondary bone tumors formed by the spreading ( metastasis ) of cancer cells from a primary tumor . They are by far the most common bone tumors in adults. In some cancers, such as breast or prostate cancer , bone metastases are a frequently occurring complication that has a significant impact on the quality of life of the affected patient as well as on the course and prognosis of the disease. In principle, any tumor that metastasizes through the bloodstream can infiltrate the bone marrow . The metastases formed in the process can be bone-forming ( osteoblastic ) or bone-degrading ( osteolytic ) or have both forms at the same time (mixed). Bone metastases cause tumor osteopathies, i.e. tumor-related bone diseases. You can influence the metabolism of the bones locally or systemically.
In addition to the lungs and liver , the skeleton is most commonly affected by cancer metastases. In many cases, bone metastases lead to considerable pain and instability in the affected bone, so that bone fractures can occur without an accident.
The diagnosis of "bone metastasis" means for the most common cancers that a cure is no longer possible. The focus of treatment is therefore in most cases purely palliative , which means that the measures taken essentially serve to improve the patient's quality of life. Administration of bisphosphonates and radiation therapy can also improve it significantly in most cases.
Dissemination and distribution
| Primary tumor | Frequency of bone metastases |
|---|---|
| Breast cancer | 50 to 85% |
| Prostate cancer | 50 to 75% |
| Bronchial carcinoma | 30 to 50% |
| Renal cell carcinoma | 30 to 50% |
| Thyroid cancer | 39% |
| Pancreatic tumor | 5 to 10 % |
| Colorectal cancer | 5 to 10 % |
| Stomach cancer | 5 to 10 % |
| Hepatocellular carcinoma | 8th % |
| Ovarian cancer | 2 to 6% |
Bone metastases are much more common than primary bone tumors such as osteosarcoma . In the United States , about 1.3 million new cases of cancer are expected each year. About 50% of these develop bone metastases in the course of the disease, which can also be detected during life. There are around 2000 cases of primary bone tumors. For example, osteosarcoma - the most common malignant primary bone tumor - there are around 200 new cases per year in Germany. In contrast, a thorough autopsy reveals bone metastases in around 70% of all patients who die from cancer.
With 210,000 cancer deaths per year in Germany, this would be around 150,000 cases of bone metastases. However, a large number of these tumor settlements remain without symptoms and are too small to be detected by means of imaging methods . Bone metastases are clinically and radiologically significant in around 15% of cancer patients. In many cases, bone metastases lead to the first symptoms of cancer.
Bone metastases have already been detected in mummies; used to be extremely rare in humans because cancer is an age-associated disease, i.e. there is a relationship between the likelihood of the disease and age. Only a small proportion of our ancestors therefore reached an age at which there is a high probability of developing cancer.
Due to the demographic change in industrialized countries, the increasing life expectancy of the population and the improved medical care, the incidence (number of new cases) of bone metastases is steadily increasing. Advances in the treatment of most cancers have led to improvements in relative survival times, which have also been correlated with an increase in the incidence of skeletal metastases. The improved cancer therapy increases the statistical probability of developing bone metastases.
The probability of a bone metastasis is very much dependent on the primary tumor and its stage. In men with the cause of death “prostate cancer”, for example, bone metastases can be detected in around 90% of those affected. Metastasis to the bones is also extremely common in breast cancer. 90% of all bone metastases come from either breast cancer, prostate cancer, bronchial cancer , kidney cancer, or multiple myeloma . Bone metastases are rare in sarcomas and - with the exception of the multiple myelomas mentioned - in lymphomas .
The mean age of the affected patients is in men in the sixth decade of life and in women - due to breast cancer - in the fifth decade.
Skeletal metastases occur frequently (multiple) in about 75% of cases. In the remaining cases they are solitary and can simulate a primary bone tumor.
Pathogenesis
metastasis
When cancer cells detach themselves from the primary tumor and migrate via the blood or lymphatic system, a small part of these cells can settle again in other organs ("form colonies") and multiply there. In the case of bone metastases, the spread occurs almost exclusively through the bloodstream.
As target organs for the colonization of tumor cells, the organs closest downstream - viewed from the primary tumor - are relatively often affected. Most of the time, the cancer cells detached from the primary tumor settle in the bone marrow via their respective arteria nutricia - the artery of a bone that supplies blood. Prostate cancer, on the other hand, metastasizes mainly through a network of veins in front of the spine and affects the lumbar spine, thighbones, pelvis, thoracic spine and ribs with decreasing frequency.
The areas of the bones in which blood formation takes place offer the tumor cells favorable growth conditions. The low flow velocity of the blood in the bones also makes it easier for the tumor cells to adhere to the vessel wall. Only then can the protein layer around the bone marrow be dissolved with the enzymes of the tumor cells and tumor cells can penetrate into the bone marrow. Bone metastases arise almost exclusively in the medullary canal.
Initially, metastasis takes place in the medullary cavity inside the bone, which is filled with red bone marrow. Then the more external areas of the substantia spongiosa and ultimately the substantia compacta are affected.
In some patients, bone metastases often do not form until many years after the primary tumor has been removed. In such cases, it is assumed that the cancer cells remain in the Dormancy tumor state for a long time and do not multiply for years before they become clinically relevant.
To a large extent, it has not yet been clarified which target organs cancer cells prefer during metastasis; According to the seed-and-soil theory , cancer cells colonize when the appropriate tumor cell ( seed ) has a particularly high affinity for the environment ( soil ) surrounding the affected organ . Proteins with good adhesive properties , such as cadherins , seem to play an important role in colonization.
Osteolytic, osteoplastic and mixed osteoplastic / osteolytic bone metastases

In the bones, the tumor cells cause local changes in the bone structure, which are caused by a disturbance of the balance in the remodeling of bone tissue . These can either be osteoplastic (bone-forming) or osteolytic (bone-degrading) or mixed osteolytic / osteoplastic. In addition, bone metastases can release various messenger substances that lead to a reduction in bone density in the entire bone system. The cells of the bone metastases are not themselves directly on the on - involved and loss of bone. These processes run via osteoclasts or osteoblasts - these are the cells that are responsible for the breakdown and build-up of the bone during bone tissue remodeling: these are addressed by the cells of the bone metastases via signal proteins. In rare cases, and only in the case of very aggressive metastases, there is a direct degradation of the bone matrix by the tumor cells, which release enzymes such as lysosomal hydrolases , peptidases and collagenases .
Osteolytic bone metastases are the most common bone metastases with a share of around 75%. The primary tumors are usually kidney, lung, breast or thyroid carcinomas. The strongly osteolytic multiple myeloma is not counted among the bone metastases in German-speaking countries.
Osteoplastic bone metastases are less common than the osteolytic variant. Their share in bone metastases is around 15%. Osteoplastic metastases mainly occur in prostate cancer, less often in other cancers. In the literature, osteoplastic metastases in breast cancer, myeloma , colorectal carcinoma, astrocytoma , glioblastoma , thymoma , carcinoid , nasopharyngeal carcinoma, Zollinger-Ellison syndrome , leptomeningeal gliomatosis and cervical carcinoma have been described.
Osteolytic and osteoplastic metastases are the two extremes of the dysregulation of bone tissue remodeling. Viewed from these two extremes, all states in between are possible in a smooth transition. The type of primary cancer has no influence on whether it is osteoplastic or osteolytic bone metastases, but can vary from patient to patient. Cancers with osteoplastic metastases, like most prostate carcinomas, also have osteolytic components which, for example, increase the risk of a pathological fracture . Most patients with bone metastases from breast cancer have osteolytic metastases, but 15 to 20% are osteoplastic.
With about 10% of the bone metastases, the mixed osteoplastic / osteolytic metastases represent the smallest group of bone metastases. In principle, all primary tumors can form colonies with mixed bone metastases. However, this is preferably the case with breast cancer and bronchial carcinoma. Osteoplastic and osteolytic metastases can also coexist on a bone.
As a reaction to the osteolysis, there is always a build-up of bone, which is visible in the X-ray as an osteoblastic border , for example , even if the bone loss predominates. In comparison, multiple myeloma - a primary cancer of the bone - is always purely osteolytic.
Interaction cancer cell with bone
Metastasis into the bones is therefore not a random process, but the result of complex molecular interactions between cancer cells and their environment: These interactions enable tumor cells to penetrate the extracellular matrix of the bone and grow in the bone .
Osteomimicry
Cells that have acquired or developed the ability to metastasize in bones express a particularly large number of genes that are related to the metabolism of the bones, that is, they have the ability to produce bone matrix proteins, with which they create the appearance of a bone cell, specifically of an osteoblast. This process is called "osteomimicry" (from the Greek osteo = 'bone' and mimicry ). The expressed proteins include the alkaline phosphatases and signal molecules that regulate the so-called crosstalk (the interaction between different transcription factors ) between osteoblasts / osteoclasts. The crosstalk between tumor cells and osteoblasts is a part of metastasis that has not yet been fully understood. It triggers an altered gene expression in the tumor cells , which promotes colonization of the bones with metastases. This cell-cell communication is an essential element of metastasis.
With the help of osteomimicry, the tumor cell secures a survival advantage in its host tissue. The immune system misses out on some of these tumor cells because they produce proteins that correspond to the protein structure of normal bone marrow cells. A very high proportion of the tumor cells that have settled are recognized and eliminated by the immune system. However, some of these cancer cells can remain undetected in their new host through evolutionary processes ( immunoediting ) and thus escape the immune system ( Immunescape ).
The osteomimicry hypothesis was first put forward in 1999 by a working group led by the US urologist Leland WK Chung and is well supported by pathological examinations.
Hypoxia as a contributing factor to bone metastases
There is an oxygen deficiency (hypoxia) in the micro-environment of the bones. The partial pressure of the oxygen pO 2 is 1 to 7%. This lack of oxygen is conducive to the growth of tumor cells in the bone metastases. Tumor cells are well adapted to hypoxic conditions. In addition, the low-oxygen environment favors the spread of tumor cells and the formation of new blood vessels ( neoangiogenesis ). The hypoxia also means that the bone metastases are highly resistant to radiation and chemotherapy, which is one of the reasons why many bone metastases are incurable. The hypoxia-induced factor HIF-1α plays an important role in hypoxia . At a high partial pressure of oxygen, HIF-1α is hydroxylated and so the target for enzymatic degradation by the Hippel-Lindau tumor suppressor . In contrast, if there is a lack of oxygen, HIF-1α is dehydroxylated and can dimerize to HIF-1β in the cell nucleus , where it mediates the transcription of hypoxia-regulated target genes. The expression of HIF-1α correlates directly with the grading of the tumor, the invasiveness and the metastasis.
Clinical appearance
| Complications from bone metastases | frequency |
|---|---|
| Bone pain | 50 to 90% |
| pathological fractures | 10 to 45% |
| spinal compression syndromes | <10% |
| Bone marrow cancer | <10% |
| Hypercalcemia | 10 to 20 % |
Pain
In many cases, pain in the spine, especially in the lumbar vertebrae, is the first symptom of cancer that involves bone. When pain occurs, the cancer is usually well advanced. Those affected often describe the pain, which does not improve even during periods of rest, as "drilling deeply" and "difficult to localize". This form of pain differs from the pain caused by instability in the spine, which occurs primarily when the spine is moved and is caused by squeezing the spinal nerves . Pain is the main factor in the decrease in quality of life in patients with bone metastases. Nerve constrictions , reduced blood flow and the release of inflammatory messenger substances (proinflammatory mediators) by the bone metastases or by "normal" cells that are in the vicinity of the bone metastases are responsible for this pain . In the latter case, the cell wall-dissolving cancer cells intervene in the self-regulation of the bone (here: balance and milieu of the bone) by releasing substances ( cytokines ) which in turn activate the osteoclasts. In addition to the breakdown of the affected bone, the activation of the osteoclasts leads to an acidic environment (low pH value ) in their environment, which causes pain in the bone. The bone formation by the osteoblasts, which counteracts bone loss but is largely uncontrolled, leads to a narrowing of the nerve endings located in the bone marrow, periosteum and in the bone matrix , which in turn is the cause of pain. The sum of these changes in the bone causes a unique mechanical and neurochemical disease process that goes beyond a pure combination of neuropathic and inflammatory pain. The neurochemical changes mean that significantly higher doses of opioids have to be administered to treat pain than, for example, inflammatory pain of a similar intensity. Treating pain is one of the main goals of palliative care for patients with bone metastases.
Pathological fractures
Less often, a bone metastasis becomes symptomatic for the first time due to a pathological fracture.
Pathological fractures are broken bones that occur without external influences due to a disease-related weakening of the bone matrix. In particular, osteolytic, but also osteoplastic bone metastases weaken the affected bone, which can then be overwhelmed by even slight mechanical loads and break. These breaks can occur in everyday situations such as getting up from an armchair or moving a patient. In osteoplastic bone metastases, the bone density is increased, but the bone strength is reduced due to the completely unstructured structure. They can lead to stiffening in the spine area.
Pathological fractures lead to far-reaching complications and have a significant impact on quality of life and prognosis. The average life expectancy may be reduced by several months. With bisphosphonates this type of bone fracture can be avoided in many cases.
The ribs or vertebral bodies are usually affected by bone fractures. Fractures in the long bones, especially in the neck of the thigh bone (" femoral neck fracture "), have a particularly high morbidity and are the main reason for surgical treatment. Fractures of the vertebral bodies can lead to spinal compression syndromes.
- Recordings of pathological fractures
Spinal Compression Syndromes
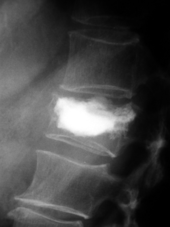
Compression fractures of one or more vertebral bodies can lead to compression of the spinal cord or the cauda equina ( cauda equina syndrome ) and are dreaded complications of bone metastasis. These spinal compression syndromes ( cross-sectional syndromes ) usually manifest themselves as severe back pain as well as motor and sensory deficits in the lower extremities. Control of the bladder and rectum can also be affected by the compression.
Spinal compression syndrome occurs in around 5% of all cancer patients. The approximate proportion of the affected vertebrae is 70% in the thoracic vertebrae , 20% in the lumbar vertebrae and 10% in the cervical vertebrae . In 4 to 22% of cases of spinal compression syndromes, it is the first symptom of the causative cancer. This is particularly the case with lymphoma, renal cell carcinoma and bronchial carcinoma.
The average survival time of patients with spinal compression syndrome caused by bone metastases is two to six months.
The period between the occurrence of neurological deficits and their treatment should be a maximum of 24 hours. Treatments are purely palliative, for example with radiation therapy, high doses of glucocorticoids such as dexamethasone and surgical fixation with implants .
Hypercalcemia
Hypercalcaemia can develop in around 10 to 20% of all patients with bone metastases. These have an increased number of osteoclasts, which cause increased bone breakdown, as a result of which the calcium ions bound in the bones are released and pass into the blood. The free calcium ions in the plasma that are not bound to proteins can be acutely life-threatening. The increased blood calcium level can be effectively reduced by drugs such as bisphosphonates or glucocorticoids . In acute cases, the calcium level can be reduced quickly with the drug calcitonin .
Bone marrow cancer
A Knochenmarkkarzinose is a rare complication occurring as a result of bone metastases. In the literature, frequencies in the range of 8 to 10% are given for all cases of skeletal metastases. The metastatic cancer cells penetrate the marrow spaces of the bones, which results in a reduction or even a stop in the formation of blood-forming cells (terminal myelosuppression ). Correspondingly, bone marrow carcinosis manifests itself through symptoms such as anemia , reduced blood clotting and a disturbed immune system (increased susceptibility to infection). The treatment of bone marrow carcinosis is purely palliative.
diagnosis
In most cases, skeletal metastases are diagnosed by imaging tests as part of follow-up examinations for tumor diseases .
An initial differential diagnosis can be made after taking the medical history , clinical examination and evaluating a conventional X-ray image . In many cases it is possible to differentiate between the following:
- benign bone tumor that does not tend to grow, such as a hemangioma
- benign tumor with a tendency to grow, such as a giant cell tumor
- primary malignant bone tumor, such as Ewing's sarcoma
- secondary malignant bone tumor (bone metastasis), with a known primary tumor or an unknown primary tumor ( CUP syndrome )
The differential diagnosis is much more difficult on the trunk skeleton, so that an additional imaging procedure is often necessary. Laboratory tests can provide additional important information to clarify the type of tumor. A tissue sample taken by biopsy (a biopsy ) can in most cases reliably and conclusively answer the question of benign or malignant as well as the type and origin of the primary tumor.
In the other cases, bone metastases are the first symptom of cancer, that is, the bone metastasis is discovered before the primary tumor. In these cases, until the primary tumor has been clarified, one speaks of a CUP syndrome ( cancer of unknown primary origin ). If the primary tumor is unknown, a detailed clinical examination of the patient is carried out. The clarification of which primary tumor caused the bone metastases is of crucial importance for further therapy planning. In purely statistical terms, male patients have a very high probability of prostate cancer as the primary tumor, which is why the prostate is usually examined intensively first. The blood levels of the tumor marker prostate-specific antigen (PSA) provide additional information. The procedure is similar for female patients. The probability of breast cancer as the primary tumor is particularly high here, which is why a detailed gynecological examination with mammography or breast sonography is usually carried out. Here the tumor marker CA 15-3 can provide further information about the diagnosis. A skeletal scintigraphy can be used to search for any additional bone metastases. It is possible that the primary tumor cannot (no longer) be localized despite complex diagnostics.
With computed tomography (CT), magnetic resonance tomography (MRT) and positron emission tomography (PET), additional imaging methods are available to search for the primary tumor. A large number of tumor markers can provide further evidence. In many cases, a biopsy provides the ultimate diagnostic confidence.
Laboratory diagnostics
In patients with bone metastases, the osteoplastic and osteolytic markers and the markers for osteoclastogenesis show an altered expression pattern. The determination of the plasma level of these markers can be used as a diagnostic aid in the case of bone metastases. Examination of the serum for certain markers associated with bone metastases does not normally provide any clear evidence for the diagnosis of bone metastases. Most laboratory parameters are too unspecific, as they can also be changed by other diseases.
In general, chemical measurements do not provide a diagnosis. However, they offer important additional information when making a diagnosis and, as tissue and / or process-specific indicators, make valuable contributions to the diagnostic “puzzle”. Since in many cases bone metastases are the first symptoms of cancer, the endocrinological parameters determined from the patient's blood can be used to confirm or invalidate the suspicion of bone metastases before more complex imaging procedures are used.
If the diagnosis is otherwise confirmed, the markers can serve as indicators for the status of the bone metastasis and can thus be used for therapy control. The markers can be used, for example, to control the effectiveness and to optimize a treatment with bisphosphonates.
For laboratory diagnostics, osteocalcin , alkaline phosphatase , the N-terminal form of procollagen type I propeptide (PINP) and the C-terminal form of procollagen type I (PICP) are important markers that indicate increased bone formation. In contrast, the two collagen fragments used carboxy-terminal type I collagen telopeptides ICTP (English cross-linked C-terminal telopeptide of type I collagen ) and β-CTX (English beta isomer of C-terminal telopeptide of type I collagen ) and the Tartrate-resistant acid phosphatase 5b (TRAcP5b) as a marker for bone resorption in bone metastases.
The levels of the various markers for bone metastases show no correlation with the degree of pain of the metastases. There is a negative correlation between the concentration of bone markers in the serum and the survival of the patient (high marker levels mean, statistically speaking, a shorter life expectancy).
PINP and PICP
With a proportion of over 90%, type I collagen is the main component of the organic bone matrix. Mature type I collagen is constantly broken down as part of normal bone remodeling. Fragments are transported to the kidneys via the bloodstream and excreted there. In the case of increased bone resorption, such as is the case with osteolytic bone metastases, the level of these fragments in the blood serum increases. In order to compensate for bone loss, repair mechanisms are activated, which are supposed to ensure sufficient bone mass. Procollagen type I, which contains amino- and carboxy-terminal precursor proteins (precursor proteins), is secreted by osteoblasts and fibroblasts . The carboxy- and amino-terminal propeptides PICP and PINP are split off by proteases during the conversion of procollagen into collagen and then released into the bloodstream. Their concentration there correlates with the extent to which new collagen type I is formed. PINP can be used as a marker in the diagnosis and follow-up monitoring of skeletal metastases, such as multiple myeloma, prostate cancer, or breast cancer. The correlation between loss of bone mass and PINP concentration has been demonstrated , for example, in patients with breast cancer after menopause . The concentration of PINP in the serum correlates with the number of bone metastases in patients with breast cancer.
ICTP
The ICTP level is increased in osteolytic and mixed osteolytic / osteoplastic bone metastases and is relatively insensitive to fluctuations in normal bone metabolism. In renal insufficiency with a glomerular filtration rate of less than 50 ml / min, the concentration of ICTP that is excreted via the kidneys is also increased. ICTP and β-CTX can serve as additional indicators for bone metastases in bronchial carcinoma.
Osteocalcin
Osteocalcin is only produced by active osteoblasts. It can be detected in both blood and urine. Osteolytic and osteoplastic bone metastases increase osteocalcin levels. However, elevated levels of this peptide hormone are not specifically observed only in bone metastases. Even with hyperparathyroidism , high-Turnover- osteoporosis , Paget's disease of bone (Paget's disease), osteomalacia , hyperthyroidism or kidney failure elevated levels are measured in serum. Osteocalcin can, for example, serve as a marker for the detection of bone metastases in differentiated thyroid carcinomas. In prostate cancer, however, it is largely useless as a marker.
Bone-specific alkaline phosphatase
Bone-specific alkaline phosphatase (BAP ) is a marker of the middle phase of bone formation that is released during the maturation of the bone matrix. BAP is a specific marker for osteogenesis and osteoplastic metastases and shows discrete to marked increases in serum levels. In prostate cancer in particular, such an increase can be observed when metastasizing into the bones. Other diseases with pronounced osteogenesis, such as osteodystrophia deformans or osteomalacia, however, also lead to increased BAP levels in the serum.
Other markers
The determination of free calcium ions in the serum can be used to diagnose hypercalcaemia as a consequence of osteolytic bone metastases. In addition to osteolytic bone metastases, hypercalcaemia can be caused by a variety of other diseases. Even the primary tumor alone can increase the calcium level in the blood by reducing the calcium excretion via the kidneys.
In patients with osteolytic metastases, increased levels of prostaglandin E2 (PGE2) are also found in the blood . The same applies to the structural protein osteopontin .
Imaging procedures
roentgen
The conventional X-ray image is of central importance for the initial diagnosis. The indication for recordings in two planes is always given when bone tumors are suspected. Osteolytic metastases are characterized by a decrease in bone density. This can be seen in the X-ray image due to the higher transparency for X-rays through a higher degree of blackening. Conversely, osteoplastic metastases show a lower degree of blackening due to the increase in bone density. Osteolytic bone metastases are much more difficult to recognize in the spine; usually only when about 50% of the total bone strength has already been lost.
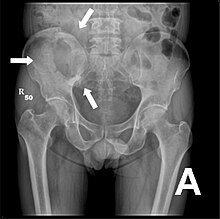
X-ray of the pelvis of a patient with prostate cancer: Multiple osteoplastic bone metastases, especially in the sacrum , but also in the iliac bone , especially on the left (i.e. on the right in the picture), on the ischial tuberosity on the left and in the proximal femur on the left. A secondary finding is a hip joint arthrosis on the right (i.e. on the left in the picture).
X-ray of the pelvic cavity osteolytic metastases. The metastases are in both thigh bones and the pelvis itself.
Skeletal scintigraphy

Metastases that are restricted to the skeletal system are very rare in colorectal cancer.
Skeletal scintigraphy is currently still the gold standard for detecting bone metastases. The procedure is relatively complex, cost-intensive and is subject to a number of diagnostic limitations. In contrast to X-ray diagnostics, the entire attack on the skeleton can be shown relatively easily with skeletal scintigraphy. A skeletal scintigraphy is usually much more sensitive than an X-ray for detecting bone metastases. The sensitivity of skeletal scintigraphy is quite high at 95%. On average, this method shows bone metastases six months before they are detected on the X-ray. In contrast, the specificity is significantly lower, since almost all tumorous and inflammatory changes in the skeleton lead to an accumulation of the radiotracer in these areas. The increased bone turnover and repair processes in the marginal area of osteolytic bone metastases result in increased storage of the radiotracer, which is visible as a hot spot in the scintigram. With bone metastases from lung or breast cancer, reactive new bone formation may not occur in rare cases. This can then be seen in the scintigram as a cold spot , an area with reduced nuclide uptake.
In the purely osteolytic multiple myeloma, however, no changes can be seen on skeletal scintigraphy. While the result of the summation of metabolic processes in the bones over a longer period of time can be seen in the X-ray image, the skeletal scintigraphy provides a snapshot of the current metabolic processes in the bones remain inconspicuous.
The bone scan is primarily for therapy monitoring of chemotherapy, radiotherapy or radionuclide therapy, for the staging (Engl. Staging ) in CUP syndrome applied and used for follow-up care for cancer.
Computed tomography
Computed tomography (CT) is usually performed after conventional x-rays and skeletal scintigraphy. In these examination procedures upstream of the CT, the areas are localized which are then to be displayed with a significantly higher resolution and better quality using CT. The CT provides information about the extent of bone destruction and the stability of the area affected by bone metastases. This information is very important in planning the treatment, especially for any surgical interventions.
The indication for a CT is therefore given before stabilizing surgical interventions (e.g. a section of the spine), before a needle biopsy (if the lesion is visible in the scintigram but not visible in the X-ray) and in the case of impending or already occurring fractures.
Magnetic resonance imaging

With magnetic resonance tomography (MRT), soft tissue in particular can be represented well in terms of images. It is the gold standard for diagnosing spinal metastases (metastases in the spine). With the MRI, further complications in the area of spinal metastases, such as infections or injuries to the intervertebral disc and ligament complex , bone marrow edema after a fresh fracture, or compression of neural structures, can be made visible. With the help of the STIR sequence ( short tau inversion recovery ) it is possible to determine the age of a fracture. T 1 weighting with gadolinium- containing contrast media , such as gadoteric acid , is particularly suitable for detecting metastases . The contrast medium accumulates particularly strongly in the metastases.
For the recurrence diagnosis , MRI is better than CT. Titanium implants, for example, produce fewer artifacts during MRI.
- Comparison of CT / MRI in osteolytic metastases of breast cancer in the spine
Sagittal reconstructed CT, displayed in the bone window. Since the patient had multiple metastases in all body regions, she could not lift her arms up for the examination, which is why the hands are also shown.
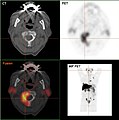
Positron Emission Tomography
- Maximum intensity projection for different tracers
Maximum intensity projection (MIP) of a PET / CT with choline . The physiological accumulation in the liver, pancreas, kidneys, bladder, spleen, bone marrow and the salivary glands can be clearly seen. The bone metastasis is in the pubic bone on the left.
PET / CT in MIP with 18 fluoro-deoxyglucose (FDG) in metastatic breast cancer. Primary tumor in the right breast. Lymph node metastases in the mediastinum and lung metastases . The bone metastases can be better delineated in the examination with 18 F-NaF (next video).
PET / CT in MIP with 18 F sodium fluoride (NaF) in metastatic breast cancer. Bone metastases in the skull, spine, pelvis, ribs, left collarbone and right thigh. The same patient as in the FDG video
Bone metastases of various tumor diseases can be detected very well with PET / CT. The procedure is more sensitive than SPECT and considerably more sensitive than skeletal scintigraphy , but it is also more complex and therefore more expensive. Depending on the tumor entity, different tracers are used, but mostly FDG and sodium fluoride , in prostate cancer also 18 F-choline. Metabolic activity of the tumor can be visualized with the help of the tracer FDG, the reaction of the bone to the tumor with the sodium fluoride PET / CT.
- F-18 choline PET / CT
Osteolytic bone metastasis in the second cervical vertebra (axis) of a metastatic prostate carcinoma
CT of a prostate cancer metastasis in the left pubic bone (right in the picture)
Bone marrow biopsy
A bone marrow biopsy can provide essential information about the etiology of the bone metastasis, especially if the primary tumor is unknown . From the cells of the metastasis taken by biopsy, an allocation to the primary tumor can be made by a pathohistological examination. A bone marrow biopsy may be indicated even if the primary tumor is known. In these cases, it serves to confirm the diagnosis and to rule out a second carcinoma.
The biopsy also provides important information for the differential diagnosis of whether it is a bone metastasis or a primary bone tumor. Since a biopsy can provide artifacts in a subsequent imaging diagnosis, it is usually always performed after the imaging procedure has been completed.
With strongly dedifferentiated cells, no assignment to a primary tumor can be made, even histopathologically.
therapy
For the most common bone metastases caused by breast, prostate or bronchial carcinoma, curative (curative) treatment is no longer possible in most cases. In addition, there is currently no effective treatment method for bone metastases. Bisphosphonates and the RANKL antibody denosumab increase the quality of life, but do not extend the life span of the patient.
In contrast to metastases in the lungs or liver, the survival time with bone metastases is relatively long. You may therefore be able to determine the morbidity of many cancers over many years based on their symptoms. The treatment of bone pain is one of the main palliative treatment goals. In some publications, the administration of calcitonin is described as pain-relieving. The active ingredient is either injected under the skin or administered as a nasal spray . More recent studies come to the conclusion that calcitonin is not suitable for the pain therapy of bone metastases.
The cells of bone metastases behave largely like the cells of the primary tumor from which they arose. Many therapeutic measures that are effective in the primary tumor also have an effect on the bone metastases. This form of treatment, to which “ hormone therapy ” (better: anti- hormone therapy ) and chemotherapy belong, is directed against the cancer itself and not specifically against bone metastases. Other forms of therapy are used specifically to combat bone metastases. These include radiation therapy, bisphosphonate therapy and surgical interventions.
radiotherapy
The use of radiation therapy to treat bone metastases is purely palliative. The cancer is not cured, but about 70% of patients treated in this way have significant pain relief. According to a meta-study from 2000, 41% of the treated patients achieved a pain reduction of at least 50% for at least one month. A third of the patients were completely pain-free after treatment. About 70% of the osteolytic metastases recalcify after the irradiation. The full load-bearing capacity of the bone is achieved again after about six months in the case of larger lesions. Usually relatively low doses of radiation, in the range of 10 to 40 gray , are used. This dose is usually divided into 15 to 18 smaller divided doses. The radiation can be carried out daily on an outpatient basis and lasts up to four weeks. Due to the low dose, serious side effects are very rare. Private life is not compromised and treatment is not stigmatizing . The hair only falls out in the directly irradiated areas. Nausea and weakness usually only occur with radiation to multiple bone metastases. Only the markings of the field boundaries are visible on the skin. The relatively low dose mainly inhibits the growth of osteoclasts and reduces inflammation in the bone tissue. Before / during radiation therapy, a nephrologist should always be consulted in order to ensure optimal protection of the kidneys, which are often pushed to the limit of their ability by an increased accumulation of degradation products from radiation treatment, especially in older people. If kidney protection is neglected, kidney failure and dependency on dialysis for the rest of an already arduous life can result.
Palliative radionuclide therapy
In palliative radionuclide therapy, the patient is injected with radiopharmaceuticals . As with radiation therapy, radionuclide therapy inhibits bone remodeling and inflammation in the area of the bone metastases. The effectiveness of radionuclide therapy for bone metastases in breast cancer and prostate cancer has been demonstrated in several studies. The response rate is around 70% of the treated patients. Complete freedom from pain is achieved in around 30% of patients. Pain relief begins about 48 hours after treatment with Rhenium -186 and Samarium -153 and lasts between one and twelve months. In the first two to four days, about 10 to 30% of patients experience an initial worsening of pain ( pain flare ) lasting an average of three days . Depending on the primary tumor, other radiopharmaceuticals besides rhenium-186 and samarium-153 can be used. With 223 Ra chloride (Alpharadin, trade name: Xofigo®) an α-emitter is also approved for the treatment of symptomatic bone metastases of castration-resistant prostate carcinomas.
Bisphosphonates
Bisphosphonates are - together with the RANKL antibody denosumab - the only drugs that are specifically used to treat bone metastases. Bisphosphonates were previously considered the "gold standard". However, their use is purely palliative and not curative.
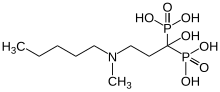
Bisphosphonates were originally developed to treat osteoporosis . They stabilize brittle bones by bonding particularly tightly to the bone tissue. This makes it difficult for the osteoclasts to break down the bone. Bisphosphonates are generally well tolerated and can be administered with hormone or chemotherapy. Modern bisphosphonates, such as zoledronate , are fast and long-lasting. Zoledronate is administered by infusion. Orally available bisphosphonates include clodronate and ibandronate . They can be easily taken in tablet form, are very well tolerated by the kidneys and are well suited for long-term therapy. The various bisphosphonates differ only marginally in their effectiveness. Bisphosphonates also reduce bone pain caused by the metastases.
Bisphosphonate-associated bone necrosis in the jaw area ( osteonecrosis of the jaw ) is a side effect of bisphosphonates that has been described since 2003. This bone change is characterized by avascular bone necrosis that is limited to the jawbone.
The possibility of whether the administration of bisphosphonates before the detection of bone metastases has a preventive or even anti-tumor effect is controversial. The studies available so far do not yet provide a uniform picture. In vitro , zoledronate has the property of suppressing bone metastases, reducing cell proliferation and increasing the rate of apoptosis .
RANKL antibodies
For the first time, the fully human monoclonal antibody denosumab offers the possibility of causally intervening in the pathophysiological mechanism of osteoclast- mediated bone destruction, which is why it is referred to as the new "gold standard". Denosumab and a. is approved for the prevention of skeletal-related complications (SRE) (pathological fracture, radiation of the bone, spinal cord compression or surgical interventions on the bone) in adults with bone metastases due to solid tumors. The mean time to onset of the first SRE was 27.6 months for denosumab and 19.4 months for the bisphosphonate zoledronic acid . Denosumab interrupts the transmission of signals between the osteoblasts and the bone-degrading osteoclasts. It takes on the role of osteoprotegerin , which is the natural opponent of RANKL . This interrupts the transmission of signals to the osteoclast and thus also the vicious circle of bone destruction. Because when the osteoclast no longer receives commands, it cannot continue to break down the bone. The skeleton, which is attacked and threatened by metastases, is protected by reducing the formation and activation of bone-degrading cells.
Denosumab is also approved for the treatment of osteoporosis (bone loss) in postmenopausal women with an increased risk of fractures. Denosumab significantly reduces the risk of vertebral, non-vertebral and hip fractures. The treatment of bone loss in connection with hormone ablation in men with prostate cancer with an increased risk of fractures is also part of the approval. Prolia significantly reduces the risk of vertebral fractures in men with prostate cancer taking hormone ablation therapy.
Anti-hormone therapy
Some types of tumors require certain sex hormones to grow . The production of these hormones can be inhibited by anti-hormone therapy or hormone withdrawal therapy. The growth of the primary tumor and its metastases are also inhibited. A cure is not possible with anti-hormone therapy. In patients with tumors that respond to this form of therapy, however, growth can be halted over longer periods of time (many months to years). In addition, the symptoms caused by the tumor are alleviated. As the cancer progresses, after a while, most tumor cells no longer need the sex hormones for their further growth. The tumor is then "hormone-deaf" (hormone-refractory) and anti-hormone therapy is therapeutically ineffective.
chemotherapy
With chemotherapy, cells are inhibited in their growth. Strongly proliferating cells, which also include cancer cells in the bone metastases, are particularly damaged - but also healthy cells with a high division rate. For example, the cells in the hair follicles are disturbed in their proliferation and differentiation , which can lead to hair loss ( alopecia ).
The choice of chemotherapeutic agent is essentially determined by the type of tumor and its growth rate as well as the general condition of the patient. Positive synergistic effects can occur between chemotherapy and hormone treatment .
surgery
Surgical interventions on bones affected by metastases are part of an overall concept. In addition to an often only palliative approach, curative approaches, e.g. B. in renal cell carcinoma, possible. The general condition of the patient is of particular importance. Not everything that is surgically feasible will be realized. The operative measure should bring a profit in relation to the overall disease. The expected survival time of the patient should be longer than the follow-up treatment time ( convalescence ) required by the operation . In many cases, even the best possible treatment does not extend the life expectancy of a patient with bone metastases. The general condition of the patients is extremely poor because of the disease and the consequences of the therapy. The complication rate and mortality are therefore considerably higher than in other patient groups. In individual cases, especially with metastases from renal cell carcinoma, there is also a curative opportunity with surgical therapy.
There is an acute need for action, especially in the case of pathological fractures , i.e. fractures of diseased bones without the use of external force, if the long bones or areas of the pelvis near the hip are involved. Surgical intervention as prompt as possible is also indicated in the case of instability of the spine caused by bone metastases, which can lead to neurological failures, as well as in the case of spinal or radicular nerve compression. The spinal cord can be given space from behind (dorsal) and the spine can be stabilized with metal implants. Vertebral bodies and parts of the vertebral body can also be removed in the front section of the spine. Metal baskets are mostly used here. Minimally invasive procedures such as vertebrolasty or kyphoplasty are also possible. The complication rate in surgical interventions on the spine is comparatively high compared to the same interventions without tumor disease. Complications occur in 6 to 9% of patients. Another intervention due to local recurrence is necessary in at least 5% of cases.
Bones that are likely to fracture from progressive osteolysis also require preventive surgery. If osteolysis has progressed to more than 50% in a long bone, there is a high probability of a possible pathological fracture. Especially with metastases in the trochanter massif there is a high risk of fractures.
If possible, surgical intervention removes the metastasis completely from the affected bone. The area of the long tubular bones near the joint is usually provided with a tumor endoprosthesis for stabilization. If the metastasis is not in the vicinity of a joint, the long bone can be strengthened using composite osteosynthesis or a diaphyseal prosthesis.
Therapy prospects
Initial therapeutic success results in therapy-resistant bone metastases after some time . Various new therapeutic methods are currently being developed or are being clinically tested to improve this situation.
Ablative procedures
With open surgery, the survival rate of elderly patients and those in poor general condition is very low. Small invasive interventions , on the other hand, usually cause less pain and lead to a faster recovery of the patient.
Various image-guided ablative methods for treating bone metastases have been developed in recent years. These include radio frequency ablation (RFA), cryotherapy , high intensity focused ultrasound (HIFU), and laser ablation . These methods are based on the local destruction of tumor cells by purely physical action, mainly heat, or in the case of cryotherapy, cold. The instruments are usually guided to the location of the metastases with the aid of imaging methods, for example by sonography, CT, MRT or fluoroscopy ( image-guided percutaneous ablation ). These procedures can also be combined with minimally invasive vertebroplasty procedures - for example kyphoplasty - in which liquid bone cement is injected into the damaged bones for stabilization. In patients for whom conventional surgical intervention is not possible, high-frequency ablation offers an alternative to the treatment of skeletal metastases.
With this method, only a limited number of bone metastases can be treated, which is why the particularly painful lesions are preferably treated with a larger number.
These procedures are occasionally used for the palliative treatment of bone metastases. So far, there are insufficiently meaningful data on the effectiveness and sustainability from smaller clinical studies, the results of which - especially with high-frequency ablation - are very promising.
Active ingredients can also be applied locally to bone metastases using image guidance. An example is ethanol in percutaneous ethanol ablation .
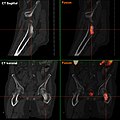
- CT images of a radio frequency ablation (RFA)
Drug development
With a better understanding of the molecular biological relationships that lead to metastasis in the bones and the associated destructive processes, some potential new active ingredients could be developed that may also increase the mean survival time. However, these active ingredients are still in clinical trials.
The monoclonal antibody denosumab, for example, provides promising approaches (see 5.4 RANKL antibodies ).
Other potential active ingredients include odanacatib , everolimus , atrasentan and the monoclonal antibody MCS110, which is directed against M-CSF ( macrophages colony-stimulating factors ).
forecast
As with all spreading (metastatic) cancers, the overall prognosis for bone metastasis is poor. Cancer is usually no longer curable. In principle, the prognosis for bone metastases is strongly dependent on the primary tumor , which is why this is the most important prognostic factor. Metastases behave largely like the primary tumor. Consequently, primary tumors that are difficult to treat have (bone) metastases that are difficult to treat. An example of this is bronchial carcinoma. In tumors with very good therapeutic results, even in advanced stages with distant metastases, bone metastases can in many cases be treated curatively, ie it leads to a complete recovery. Examples of this are above all testicular cancer and follicular thyroid carcinoma .
In patients with breast or prostate cancer, the mean survival time after the initial diagnosis of “bone metastasis” is 12 to 18 months, while it is only three months in patients with lung cancer. Patients who have metastasized only in the bones can survive for ten or more years.
The number of bone metastases, whether they occur individually or in multiple, has a significant impact on the prognosis - at least in the case of breast cancer and renal cell carcinoma. If the tumor metastasizes to other organs, the prognosis is determined solely by these tumor settlements and is considerably worsened overall. The bone metastases then hardly have any significant influence on life expectancy. Although the location of the metastasis in the skeleton has no influence on the prognosis, it does have a considerable influence on the possibilities of surgical interventions. The age of the patient does not significantly influence the prognosis either. In prostate cancer, bone metastases are the primary cause of death.
Another important prognostic parameter is the so-called Karnofsky index , which describes the general performance of cancer patients.
Bone metastases in veterinary medicine

Bone metastases are very old in evolutionary terms. They can already be detected radiologically in fossils such as dinosaur bones.
The incidence of bone metastases is significantly lower in dogs than in humans. In breast cancer, for example, only about 10% of the diseased animals with distant metastases in the bones are affected. In these cases, the lumbar vertebrae and the pelvic bones are essentially affected. Other tumors that typically proliferate (metastasize) the dog's bones are prostate, urethral , and bladder cancers, and malignant histiocytosis . Primary bone tumors of the osteosarcoma type, which are much more common in dogs - especially in large breeds - often metastasize into the bones. With the increasing use of chemotherapeutic agents in the therapy of cancers in dogs, the metastatic behavior of the tumors is evidently changed in such a way that more bone tumors are found.
In cats , tumor metastasis to the skeleton is extremely rare. Tumor diseases that occasionally do this include hemangiosarcoma , prostate carcinoma, osteosarcoma, and urothelial carcinoma of the urinary system.
For research into metastasis of the skeletal system, its prevention and therapy, the color mouse model organism , especially the nude mouse , is used. In mice with an immune deficiency to human cancer cells can bring that result after a few weeks to bone metastases.
further reading
General
- D. Kardamakis, V. Vassiliou, E. Chow: Bone Metastases - A Translational and Clinical Approach. Springer, 2009, ISBN 1-4020-9818-9 restricted preview in the Google book search
- C. Jasmin, R. Capanna, et al. a. (Ed.): Textbook of bone metastases. John Wiley and Sons, 2005, ISBN 0-471-87742-5 limited preview in Google Book Search
- M. Joerger, M. Gnant (Ed.): Prevention of Bone Metastases. Springer, 2012, ISBN 3-642-21891-1 .
Pathogenesis
- LJ Suva, C. Washam, RW Nicholas, RJ Griffin: Bone metastasis: mechanisms and therapeutic opportunities. In: Nature Reviews Endocrinology . Volume 7, number 4, April 2011, pp. 208-218, doi : 10.1038 / nrendo.2010.227 . PMID 21200394 . PMC 3134309 (free full text). (Review).
- MN Thobe 1, RJ Clark, RO Bainer, SM Prasad, CW Rinker-Schaeffer: From Prostate to Bone: Key Players in Prostate Cancer Bone Metastasis. In: Cancers 3, 2011, pp. 478–493, doi : 10.3390 / cancers3010478 (review in Open Access )
- KC Nannuru, RK Singh: Tumor-stromal interactions in bone metastasis. In: Current osteoporosis reports Volume 8, Number 2, June 2010, pp. 105-113, doi : 10.1007 / s11914-010-0011-6 . PMID 20425618 . (Review).
- MS Virk, JR Lieberman: Tumor metastasis to bone. In: Arthritis research & therapy Volume 9 Suppl 1, 2007, p. S5, doi : 10.1186 / ar2169 . PMID 17634144 . PMC 1924520 (free full text). (Review).
- LA Kingsley, PG Fournier et al. a .: Molecular biology of bone metastasis. In: Molecular Cancer Therapeutics Volume 6, Number 10, October 2007, pp. 2609-2617, doi : 10.1158 / 1535-7163.MCT-07-0234 . PMID 17938257 . (Review).
- ET Keller and LWK Chung (Eds.): The biology of skeletal metastases. Volume 118, Springer, 2004, ISBN 1-4020-7749-1 limited preview in the Google book search
- PJ Kostenuik: Revisiting the seed and soil theory of bone metastasis: new tools, same answer. In: Journal of musculoskeletal & neuronal interactions Volume 4, Number 4, December 2004, pp. 375-376, PMID 15758267 . (Review).
- TA Guise: Molecular mechanisms of osteolytic bone metastases. In: Cancer Volume 88, Number 12 Suppl, June 2000, pp. 2892-2898, PMID 10898330 . (Review).
- JM Chirgwin, TA Guise: Molecular mechanisms of tumor-bone interactions in osteolytic metastases. In: Critical reviews in eukaryotic gene expression Volume 10, Number 2, 2000, pp. 159-178, PMID 11186331 . (Review).
diagnosis
- D. Hellwig, BJ Krause, H. Schirrmeister, M. Freesmeyer: Skeletal diagnostics using 18F sodium fluoride PET and PET / CT. In: Nuklearmedizin Volume 49, Number 5, 2010, pp. 195-201, doi : 10.3413 / nukmed-0343 . PMID 20838734 .
- CM Costelloe, HH Chuang, JE Madewell, NT Ueno: Cancer Response Criteria and Bone Metastases: RECIST 1.1, MDA and PERCIST. (PDF; 944 kB) In: Journal of Cancer Volume 1, 2010, pp. 80-92, PMID 20842228 . PMC 2938069 (free full text).
- AM Davies, M. Sundaram, SLJ James: Imaging of Bone Tumors and Tumor-Like Lesions. Springer, 2009, ISBN 3-540-77982-5 limited preview in the Google book search
- R. Dichtel: Magnetic resonance tomographic whole-body screening of bone metastases in children - comparison with skeletal scintigraphy. (PDF; 684 kB) Dissertation, Ludwig Maximilians University of Munich, 2005.
therapy
- A. Lipton: Implications of bone metastases and the benefits of bone-targeted therapy. In: Seminars in Oncology Volume 37 Suppl 2, October 2010, pp. S15 – S29, doi : 10.1053 / j.seminoncol.2010.10.002 . PMID 21111244 . (Review).
- G. Bauman, M. Charette, R. Reid, J. Sathya: Radiopharmaceuticals for the palliation of painful bone metastasis-a systemic review. In: Radiotherapy and Oncology Volume 75, Number 3, June 2005, pp. 258-270, PMID 16299924 . (Review).
- G. Singh, SA Rabbani: Bone metastasis: experimental and clinical therapeutics. Humana Press, 2005, ISBN 1-58829-403-X limited preview in Google Book Search
- P. Schmid: Supportive therapy of bone metastases. UNI-MED-Verlag, 2005, ISBN 3-89599-906-7
- EB Silberstein, L. Eugene, SR Saenger: Painful osteoblastic metastases: the role of nuclear medicine. In: Oncology (Williston Park, NY) Volume 15, Number 2, February 2001, pp. 157-163, PMID 11252931 . (Review).
Web links
- Bone metastases. German Cancer Society
- Bone metastases: Information for patients and interested parties - background, diagnosis, treatment and living with the disease , cancer information service of the German Cancer Research Center (DKFZ), Heidelberg. September 6, 2012. Last accessed September 4, 2014.
- www.leben-mit-knochenmetastasen.de Information for patients and relatives
- Detailed presentation of surgical therapy and prognosis of bone metastases
- Dialogue between bones and tumor cells. Professional association of specialists in orthopedics and trauma surgery
Individual evidence
- ↑ a b C. P. Adler: Bone Diseases. Edition 3, Springer, 2005, ISBN 3-540-21962-5 , pp. 418-429. limited preview in Google Book search
- ↑ a b c d A. Pelz: Retrospective investigations on the cytological and histological diagnosis of bone marrow metastases in the test material of the Institute for Pathology of the Bad Saarow Clinic in the period from 1993 to 2006. (PDF; 2 MB) Dissertation, Charité - Universitätsmedizin Berlin, 2009
- ↑ a b c d e f g B. Krempien: The development of bone pain in bone metastases and their treatment with bisphosphonates. In: HH Bartsch, W. Hornstein (Ed.): Interdisciplinary pain therapy for tumor patients. Karger Publishers, 1998, ISBN 3-8055-6594-1 , limited preview in Google Book Search
- ↑ H. Yasuda, N. Shima, N. Nakagawa, K. Yamaguchi, M. Kinosaki, S. Mochizuki, A. Tomoyasu, K. Yano, M. Goto, A. Murakami, E. Tsuda, T. Morinaga, K. Higashio, N. Udagawa, N. Takahashi, T. Suda: Osteoclast differentiation factor is a ligand for osteoprotegerin / osteoclastogenesis-inhibitory factor and is identical to TRANCE / RANKL. In: Proceedings of the National Academy of Sciences of the United States of America Volume 95, Number 7, March 1998, pp. 3597-3602, PMID 9520411 . PMC 19881 (free full text).
- ↑ a b G. D. Roodman: Mechanisms of bone metastasis. In: Discovery medicine Volume 4, Number 22, June 2004, pp. 144-148, PMID 20704976 . ( Open access )
- ↑ a b c d e f g h i I. J. Diel, H. Seegenschmiedt: Therapy of skeletal metastases. In: H.-J. Schmoll, K. Höffken, K. Possinger (Eds.): Compendium of internal oncology. ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. (PDF; 792 kB) Springer 2006, ISBN 978-3-540-20657-6 , pp. 994-1014. limited preview in Google Book search
- ↑ a b G. Layer: Skeletal Metastases. In: J. Freyschmidt, A. Stäbler: Handbuch diagnostic radiology. Springer, 2005, pp. 327-338, ISBN 978-3-540-26388-3 doi : 10.1007 / 3-540-26388-8_11
- ↑ a b c W. K. Hong, RC Bast, W. Hait, u. a .: Holland-Frei: Cancer Medicine. Volume 8, BC Decker, 2009, ISBN 1-60795-014-6 , limited preview in Google Book Search
- ^ JR Neff: Metastatic disease to bone. In: MM Lewis (ed.): Musculoskeletal oncology: a multi-disciplinary approach. WB Saunders, 1992, pp. 377-399, ISBN 0-7216-5771-0
- ^ HL Abrams, R. Spiro, N. Goldstein: Metastases in carcinoma; analysis of 1000 autopsied cases. In: Cancer Volume 3, Number 1, January 1950, pp. 74-85, PMID 15405683 .
- ↑ M. Campanacci: Anonymous bone and soft tissue tumors. In: Bone metastases from carcinomas. Springer, Vienna New York, 1990, pp. 677-679.
- ↑ a b c d H. R. Dürr, HJ Refior: Prognosis of skeletal metastases. ( Memento of the original from June 6, 2011 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. In: Der Orthopäde Volume 27, Number 5, May 1998, pp. 294-300, PMID 9646321 .
- ^ A b R. A. Ruggiero, OD Bustuoabad, P. Chiarella, J. Bruzzo, RP Meiss: On the biological Significance of Tumor Growth. In: D. Kimura (Ed.): Cell growth processes - New Research. Nova Publishers, 2008, ISBN 1-60456-132-7 , p. 91. Limited preview in Google Book Search
- ↑ ME Kricun: Edward BD Neuhauser Lecture. Paleoradiology of the prehistoric Australian aborigines. In: American Journal of Roentgenology . Volume 163, Number 2, August 1994, pp. 241-247, doi: 10.2214 / ajr.163.2.8037007 , PMID 8037007 .
- ↑ Y. Gorina, D. Hoyert, H. Lentzner, M. Goulding: Trends in Causes of Death among Older Persons in the United States. (PDF; 359 kB) In: Aging Trends. No 6, National Center for Health Statistics, 2006, based on data from Deaths: Leading Causes of 2002. NVSR Vol. 53, no.17.
- ^ Robert Koch Institute (ed.): Cancer in Germany 2005/2006 - Frequencies and Trends. ( Memento of the original from November 3, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. 7th edition, 2010.
- ↑ DM Sciubba, ZL Gokaslan: Diagnosis and management of metastatic spine disease. In: Surgical Oncology Volume 15, Number 3, November 2006, pp. 141-151, doi: 10.1016 / j.suronc.2006.11.002 . PMID 17184989 . (Review).
- ↑ a b c S. Freynik: balloon kyphoplasty of pathological fractures of the vertebral bodies with vertebral tumor metastases. Prospective study with 65 patients over a two year follow-up period. (PDF; 5.2 MB) Dissertation, Medical Faculty Charité - Universitätsmedizin Berlin, 2009
- ↑ L. Bubendorf, A. Schöpfer, U. Wagner, G. Sauter, H. Moch, N. Willi, TC Gasser, MJ Mihatsch: Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. In: Human pathology Volume 31, Number 5, May 2000, pp. 578-583, PMID 10836297 .
- ↑ I. Roato, P. D'Amelio, E. Gorassini, A. Grimaldi, L. Bonello, C. Fiori, L. Delsedime, A. Tizzani, A. De Libero, G. Isaia, R. Ferracini: osteoclasts are active in bone forming metastases of prostate cancer patients. In: PLoS ONE Volume 3, Number 11, 2008, p. E3627, doi: 10.1371 / journal.pone.0003627 . PMID 18978943 . PMC 2574033 (free full text).
- ↑ CP Adler, GW Herget, M. Uhl: Radiological diagnosis of bone diseases. Springer, 2004, ISBN 3-540-20465-2 , p. 166. Restricted preview in the Google book search
- ↑ a b c d H. R. Dürr, V. Jansson, PU Tunn: Differentiated therapy of metastatic lesions of the bone. In: Arthritis and Rheuma 3, 2007, pp. 162-168.
- ↑ a b L. A. Liotta, EC Kohn: Holland-Frei: Invasion and Metastases. 5th edition, BC Decker, 2000.
- ↑ T. Peters: Incidence of bone metastases in the initial diagnosis of prostate cancer depending on the prostate-specific antigen. Dissertation, Westfälische Wilhelms-Universität Münster, 2006. DNB 991641671/34
- ^ RL Carter: Patterns and mechanisms of bone metastases. In: Journal of the Royal Society of Medicine Volume 78 Suppl 9, 1985, pp. 2-6, PMID 4045915 . PMC 1289525 (free full text).
- ↑ H. Noltenius, D. Wolter: On the general pathology of tumor metastases. In: H. Remé (Ed.): Osteolysen: pathological fractures. Thieme, 1982, ISBN 3-13-636501-1 , pp. 2-14.
- ↑ a b c d e f g S. Braun: Surgical therapy and prognosis in patients with skeletal carcinoma metastases. Dissertation, Ludwig Maximilians University of Munich, 2004.
- ^ RL Carter: Patterns and mechanisms of localized bone invasion by tumors: studies with squamous carcinomas of the head and neck. In: Critical reviews in clinical laboratory sciences Volume 22, Number 3, 1985, pp. 275-315, doi : 10.3109 / 10408368509165845 . PMID 3899510 . (Review).
- ↑ a b P. Clezardin, A. Teti: Bone metastasis: pathogenesis and therapeutic implications. In: Clinical & experimental metastasis Volume 24, Number 8, 2007, pp. 599-608, doi : 10.1007 / s10585-007-9112-8 . PMID 18008175 . (Review).
- ^ AF Chambers, AC Groom, IC MacDonald: Dissemination and growth of cancer cells in metastatic sites. In: Nature Reviews Cancer Volume 2, Number 8, August 2002, pp. 563-572, doi : 10.1038 / nrc865 . PMID 12154349 . (Review).
- ↑ CF Nussbaum: Function of platelets in antivascular tumor therapy using paclitaxel encapsulated in cationic liposomes. (PDF; 1.7 MB) Dissertation, Ludwig Maximilians University of Munich, 2008, p. 2.
- ^ EJ Raubenheimer, CE Noffke: Pathogenesis of bone metastasis: a review. In: Journal of Oral Pathology & Medicine Volume 35, Number 3, March 2006, pp. 129-135, doi : 10.1111 / j.1600-0714.2006.00360.x . PMID 16454807 . (Review).
- ^ R. Bartl, E. von Tresckow, C. Bartl: Bisphosphonat-Manual: Effects - Indications - Strategies. Chapter 9: Bone Metastases , Springer, 2006, ISBN 3-540-25362-9 , pp. 225–249, limited preview in the Google book search
- ↑ GS Forbes, RA McLeod, RR Hattery: Radiographic manifestations of bone metastases from renal carcinoma. In: American Journal of Roentgenology Volume 129, Number 1, July 1977, pp. 61-66, PMID 409145 .
- ↑ TA Guise, GR Mundy: Cancer and bone. In: Endocrine reviews Volume 19, Number 1, February 1998, pp. 18-54, PMID 9494779 . (Review).
- ^ Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 29-1972. In: The New England Journal of Medicine Volume 287, Number 3, July 1972, pp. 138-143, doi : 10.1056 / NEJM197207202870308 . PMID 4338086 .
- ^ MR Paling, TL Pope: Computed tomography of isolated osteoblastic colon metastases in the bony pelvis. In: The Journal of computed tomography Volume 12, Number 3, July 1988, pp. 203-207, PMID 3168541 .
- ^ JE Kingston, PN Plowman, BF Smith, NJ Garvan: Differentiated astrocytoma with osteoblastic skeletal metastases in a child. In: Child's nervous system Volume 2, Number 4, 1986, pp. 219-221, PMID 3779686 .
- ↑ AS Gamis, J. Egelhoff, G. Roloson, J. Young, GM Woods, R. Newman, Al Freeman: Diffuse bony metastases at presentation in a child with glioblastoma multiforme. A case report. In: Cancer Volume 66, Number 1, July 1990, pp. 180-184, PMID 2162242 .
- ↑ MK McLennan: Case report 657: Malignant epithelial thymoma with osteoplastic metastases. In: Skeletal radiology Volume 20, Number 2, 1991, pp. 141-144, PMID 2020863 .
- ^ N. Giordano, P. Nardi, P. Vigni, F. Palumbo, E. Battisti, C. Gennari: Osteoblastic metastases from carcinoid tumor. In: Clinical and experimental rheumatology Volume 12, Number 2, 1994 Mar-Apr, pp 228-229, PMID 8039297 .
- ↑ CC Liaw, YS Ho, NG Koon-Kwan, TL Chen, WC Tzann: Nasopharyngeal carcinoma with brain metastasis: a case report. In: Journal of neuro-oncology Volume 22, Number 3, 1994, pp. 227-230, PMID 7760099 .
- Jump up ↑ RT Pederson, DJ Haidak, RA Ferris, JS Macdonald, PS Schein: Osteoblastic bone metastasis in Zollinger-Ellison syndrome. In: Radiology Volume 118, Number 1, January 1976, pp. 63-64, PMID 1244675 .
- Jump up ↑ A. Pingi, G. Trasimeni, C. Di Biasi, G. Gualdi, G. Piazza, F. Corsi, F. Chiappetta: Diffuse leptomeningeal gliomatosis with osteoblastic metastases and no evidence of intraaxial lesions. In: AJNR. American journal of neuroradiology Volume 16, Number 5, May 1995, pp. 1018-1020, PMID 7639122 .
- ^ J. George, FM Lai: Metastatic cervical carcinoma presenting as psoas abscess and osteoblastic and lytic bony metastases. In: Singapore medical journal Volume 36, Number 2, April 1995, pp. 224-227, PMID 7676275 .
- ^ TA Guise, JJ Yin, KS Mohammad: Role of endothelin-1 in osteoblastic bone metastases. In: Cancer Volume 97, Number 3 Suppl, February 2003, pp. 779-784, doi : 10.1002 / cncr.11129 . PMID 12548575 . (Review).
- ↑ a b c d G. D. Roodman: Mechanisms of bone metastasis. In: The New England journal of medicine Volume 350, Number 16, April 2004, pp. 1655–1664, doi : 10.1056 / NEJMra030831 . PMID 15084698 . (Review).
- ^ RE Coleman, JJ Seaman: The role of zoledronic acid in cancer: clinical studies in the treatment and prevention of bone metastases. In: Seminars in oncology Volume 28, Number 2 Suppl 6, April 2001, pp. 11-16, PMID 11346860 . (Review).
- ↑ LWK Chung, W.-C. Huang, S.-Y. Sung, D. Wu, V. Odero-Marah, HE Zhau: Cancer – Host Interactions. In: LWK Chung, WB Isaacs, JW Simons (Eds.): Prostate cancer - biology, genetics, and the new therapeutics. Humana Press, 2007, ISBN 1-58829-696-2 , pp. 76-78. limited preview in Google Book search
- ↑ N. Rucci, A. Teti: Osteomimicry: How tumor cells try to deceive the bone. In: Frontiers in bioscience (Scholar edition) Volume 2, 2010, pp. 907-915, PMID 20515833 . (Review).
- ↑ a b K. Knerr, K. Ackermann, T. Neidhart, W. Pyerin: Bone metastasis: Osteoblasts affect growth and adhesion regulons in prostate tumor cells and provoke osteomimicry. In: International Journal of Cancer . Volume 111, number 1, August 2004, pp. 152-159, doi : 10.1002 / ijc.20223 . PMID 15185357 .
- ↑ K. Ackermann, W. Pyerin: Dialogues between bones and tumor cells. In: orthinform.de of April 21, 2005
- ^ GN Thalmann, MG Cecchini, A. Wetterwald, R. Schwanninger, C. Rentsch: Bone Metastases - Coincidence or Predetermined? ( Memento of the original from September 23, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 320 kB) In: UNIPRESS 120, April 2004, pp. 34–36.
- ↑ NS Fedarko, B. Fohr, PG Robey, MF Young, LW Fisher: Factor H binding to bone sialoprotein and osteopontin enables tumor cell evasion of complement-mediated attack. In: The Journal of Biological Chemistry Volume 275, Number 22, June 2000, pp. 16666-16672, doi : 10.1074 / jbc.M001123200 . PMID 10747989 .
- ↑ HT Khong, NP Restifo: Natural selection of tumor variants in the generation of "tumor escape" phenotypes. In: Nature immunology Volume 3, Number 11, November 2002, pp. 999-1005, doi : 10.1038 / ni1102-999 . PMID 12407407 . PMC 1508168 (free full text). (Review).
- ↑ KS Koeneman, F. Yeung, LW Chung: Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. In: The Prostate Volume 39, Number 4, June 1999, pp. 246-261, PMID 10344214 . (Review).
- ^ A. Halas: Crosstalk of cells in bone metastasis: Molecular and cellular analysis of the mutual effects of prostate cancer cells and osteoblasts. Dissertation, University of Kaiserslautern, 2010
- ↑ M. Hirao, J. Hashimoto, N. Yamasaki, W. Ando, H. Tsuboi, A. Myoui, H. Yoshikawa: Oxygen tension is an important mediator of the transformation of osteoblasts to osteocytes. In: Journal of bone and mineral metabolism Volume 25, Number 5, 2007, pp. 266-276, doi : 10.1007 / s00774-007-0765-9 . PMID 17704991 .
- ↑ a b A. L. Harris: Hypoxia - a key regulatory factor in tumor growth. In: Nature Reviews Cancer Volume 2, Number 1, January 2002, pp. 38-47, doi : 10.1038 / nrc704 . PMID 11902584 . (Review).
- ^ JM Brown, WR Wilson: Exploiting tumor hypoxia in cancer treatment. In: Nature Reviews Cancer Volume 4, Number 6, June 2004, pp. 437-447, doi : 10.1038 / nrc1367 . PMID 15170446 . (Review).
- ↑ GL Semenza : Targeting HIF-1 for cancer therapy. In: Nature Reviews Cancer Volume 3, Number 10, October 2003, pp. 721-732, doi : 10.1038 / nrc1187 . PMID 13130303 . (Review).
- ↑ a b c d e f L. K. Dunn, KS Mohammad, PG Fournier, CR McKenna, HW Davis, M. Niewolna, XH Peng, JM Chirgwin, TA Guise: Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. In: PloS one Volume 4, number 9, 2009, p. E6896, doi : 10.1371 / journal.pone.0006896 . PMID 19727403 . PMC 2731927 (free full text).
- ↑ H. Zhong, AM De Marzo, E. Laughner, M. Lim, DA Hilton, D. Zagzag, P. Buechler, WB Isaacs, GL Semenza , JW Simons: Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. In: Cancer Research Volume 59, Number 22, November 1999, pp. 5830-5835, PMID 10582706 .
- ↑ a b c D. A. Wölke: Results of radiation therapy for painful skeletal metastases. Dissertation, Westfälische Wilhelms-Universität Münster, 2009. DNB 99711424X / 34
- ^ A b S. Mercadante, F. Fulfaro: Management of painful bone metastases. In: Current opinion in oncology Volume 19, Number 4, July 2007, pp. 308-314, doi : 10.1097 / CCO.0b013e3281214400 . PMID 17545792 . (Review).
- ↑ P. Honore, PW Mantyh: Bone cancer pain: from mechanism to model to therapy. In: Pain medicine (Malden, Mass.) Volume 1, Number 4, December 2000, pp. 303-309, PMID 15101876 .
- ↑ I. Roato, M. Grano, G. Brunetti, S. Colucci, A. Mussa, O. Bertetto, R. Ferracini: Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. In: The FASEB journal Volume 19, Number 2, February 2005, pp. 228-230, doi : 10.1096 / fj.04-1823fje . PMID 15550550 .
- ^ HA Harvey, LR Cream: Biology of bone metastases: causes and consequences. In: Clinical Breast Cancer Volume 7 Suppl 1, July 2007, pp. S7-S13, PMID 17683652 . (Review).
- ↑ PW Mantyh, DR Clohisy, M. Koltzenburg, SP Hunt: Molecular mechanisms of cancer pain. In: Nature Reviews Cancer Volume 2, Number 3, March 2002, pp. 201-209, doi : 10.1038 / nrc747 . PMID 11990856 . (Review).
- ↑ M. Nagae, T. Hiraga, T. Yoneda: Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. In: Journal of bone and mineral metabolism Volume 25, Number 2, 2007, pp. 99-104, doi : 10.1007 / s00774-006-0734-8 . PMID 17323179 .
- ↑ DB Mach, SD Rogers, MC Sabino, NM Luger, MJ Schwei, JD Pomonis, CP Keyser, DR Clohisy, DJ Adams, P. O'Leary, PW Mantyh: Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur . In: Neuroscience Volume 113, Number 1, 2002, pp. 155-166, PMID 12123694 .
- ↑ P. Honore, SD Rogers, MJ Schwei, JL Salak-Johnson, NM Luger, MC Sabino, DR Clohisy, PW Mantyh: Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. In: Neuroscience Volume 98, Number 3, 2000, pp. 585-598, PMID 10869852 .
- ↑ L. Doré-Savard, V. Otis, K. Belleville, M. Lemire, M. Archambault, L. Tremblay, JF Beaudoin, N. Beaudet, R. Lecomte, M. Lepage, L. Gendron, P. Sarret: Behavioral, medical imaging and histopathological features of a new rat model of bone cancer pain. In: PloS one Volume 5, Number 10, 2010, p. E13774, doi : 10.1371 / journal.pone.0013774 . PMID 21048940 . PMC 2966439 (free full text).
- ↑ RJ Papac: Bone marrow metastases. A review. In: Cancer Volume 74, Number 9, November 1994, pp. 2403-2413, PMID 7922993 . (Review).
- ↑ a b c d e H. J. Helling: Therapy of symptomatic bone metastases. In: UB Hankemeier (ed.): Tumor pain therapy . Springer, 2004, ISBN 3-540-20431-8 , pp. 167f. limited preview in Google Book search
- ^ S. Bottiglieri, V. Adams, KM Smith: Pharmacologic prevention of skeletal-related events in cancer patients. In: Orthopedics Volume 33, Number 8, August 2010, pp. 577-580, doi : 10.3928 / 01477447-20100625-20 . PMID 20704155 . (Review).
- ↑ L. Costa, PP Major: Effect of bisphosphonates on pain and quality of life in patients with bone metastases. In: Nature Clinical Practice Oncology Volume 6, Number 3, March 2009, pp. 163-174, doi : 10.1038 / ncponc1323 . PMID 19190592 . (Review).
- ↑ a b c J. S. Zimmermann, MW Groß, T. Riegel, R. Engenhart-Cabillic: Spinal Compression Syndrome . In: Der Onkologe Volume 5, Number 12, 1999, pp. 1054-1061.
- ↑ a b c D. Bottke, S. Hammersen, S. Höcht and W. Hinkelbein: Spinales compression syndrome. In: Der Onkologe Volume 10, Number 4, 2004, pp. 345-350, doi : 10.1007 / s00761-004-0693-4
- ^ R. Kath, E. Aulbert, K. Höffgen: Treatment of oncological emergencies. In: E. Aulbert, F. Nauck, L. Radbruch (eds.): Textbook of Palliative Medicine. Schattauer Verlag, 2007, ISBN 3-7945-2361-X , limited preview in the Google book search
- ^ PJ Hoskin, A. Grover, R. Bhana: Metastatic spinal cord compression: radiotherapy outcome and dose fractionation. In: Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology Volume 68, Number 2, August 2003, pp. 175-180, PMID 12972313 .
- ↑ MC Chamberlain, PA Kormanik: Epidural spinal cord compression: a single institution's retrospective experience. In: Neuro-Oncology Volume 1, Number 2, April 1999, pp. 120-123, PMID 11550307 . PMC 1920751 (free full text). (Review).
- ↑ F. Lumachi, A. Brunello, A. Roma, U. Basso: Cancer-induced hypercalcemia. In: Anticancer Research Volume 29, Number 5, May 2009, pp. 1551-1555, PMID 19443365 . (Review).
- ↑ AA Kurth, L. Hovy, T. Hennig: bisphosphonate bone diseases. Birkhäuser, 2001, ISBN 3-7985-1266-3 , p. 37. Restricted preview in the Google book search
- ↑ a b c d e f g h i j k l m B. Wippermann, E. Mössinger, HE Schratt, C. Krettek: Diagnostics and therapy of bone metastases. In: The trauma surgeon Volume 105, number 2, February 2002, pp. 147-160, PMID 11968542 .
- ↑ a b C. Meier, MJ Seibel: Biochemical bone remodeling markers in bone metastases. In: Onkologie 5, 2006, pp. 34-39.
- ↑ a b Q. Q. Kong, TW Sun, QY Dou, F. Li, Q. Tang, FX Pei, CQ Tu, ZQ Chen: Beta-CTX and ICTP act as indicators of skeletal metastasis status in male patients with non-small cell lung cancer. In: The International journal of biological markers Volume 22, Number 3, 2007 Jul-Sep, pp. 214-220, PMID 17922466 .
- ↑ a b c M. Lein, A. Ramankulov, C. Stephan, J. Kramer, SA Loening, K. Jung: New serum markers for the diagnosis of bone metastases in prostate cancer. In: The Urologist. Edition A Volume 46, Number 9, September 2007, pp. 1087-1088, doi : 10.1007 / s00120-007-1437-z . PMID 17628774 .
- ↑ a b H. Renz: Practical laboratory diagnostics. Walter de Gruyter, 2009, ISBN 3-11-019576-3 , p. 431. Limited preview in the Google book search
- ↑ a b A. G. Zafeirakis, GA Papatheodorou, GS Limouris: Clinical and imaging correlations of bone turnover markers in prostate cancer patients with bone only metastases. In: Nuclear medicine communications Volume 31, Number 3, March 2010, pp. 249-253, doi : 10.1097 / MNM.0b013e328335a5ed . PMID 20038858 .
- ↑ K. Jung, M. Lein, C. Stephan, K. Von Hösslin, A. Semjonow, P. Sinha, SA Loening, D. Schnorr: Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. In: International journal of cancer Volume 111, number 5, September 2004, pp. 783-791, doi : 10.1002 / ijc.20314 . PMID 15252851 .
- ↑ a b J. E. Brown, S. Sim: Evolving role of bone biomarkers in castration-resistant prostate cancer. In: Neoplasia (New York, NY) Volume 12, Number 9, September 2010, pp. 685-696, PMID 20824045 . PMC 2933689 (free full text). (Review).
- ↑ A. Berruti, M. Torta, A. Piovesan, CA Raucci, F. Orlandi, A. Panero, L. Dogliotti, A. Angeli: Biochemical picture of bone metabolism in breast cancer patients with bone metastases. In: Anticancer Research Volume 15, Number 6B, 1995, pp. 2871-2875, PMID 8669881 .
- ↑ J. Melkko, S. Kauppila, S. Niemi, L. Risteli, K. Haukipuro, A. Jukkola, J. Risteli: Immunoassay for intact amino-terminal propeptide of human type I procollagen. In: Clinical chemistry Volume 42, Number 6 Pt 1, June 1996, pp. 947-954, PMID 8665688 .
- ↑ O. Orum, M. Hansen, CH Jensen, HA Sørensen, LB Jensen, K. Hørslev-Petersen, B. Teisner: development ELISA: procollagen type I N-terminal propeptide (PINP) as in indicator of type I collagen metabolism reference interval, and hypovitaminosis D induced hyperparathyroidism. In: Bone Volume 19, Number 2, August 1996, pp. 157-163, PMID 8853860 .
- ↑ a b D. Pollmann: [ A new, automated PINP assay in comparison with established markers of bone metabolism and tumor mass in the assessment of the course of osseous metastatic breast cancer. ], Medical Faculty Charité - Universitätsmedizin Berlin, dissertation, 2007
- ↑ I. Elomaa, L. Risteli, M. Laakso, R. Lahtinen, P. Virkkunen, J. Risteli: Monitoring the action of clodronate with type I collagen metabolites in multiple myeloma. In: European Journal of Cancer Volume 32A, Number 7, June 1996, pp. 1166-1170, PMID 8758248 .
- ↑ a b M. Koizumi, J. Yonese, I. Fukui, E. Ogata: The serum level of the amino-terminal propeptide of type I procollagen is a sensitive marker for prostate cancer metastasis to bone. In: BJU international Volume 87, Number 4, March 2001, pp. 348-351, PMID 11251528 .
- ↑ R. Tähtelä, E. Thölix: Serum Concentrations of type I collagen carboxy-terminal telopeptide (ICTP) and type I procollagen carboxy-and amino-terminal propeptide (PICP, PINP) as markers metastatic bone disease of in breast cancer. In: Anticancer Research Volume 16, Number 4B, 1996 Jul-Aug, pp. 2289-2293, PMID 8694558 .
- ↑ T. Saarto, C. Blomqvist, J. Risteli, L. Risteli, S. Sarna, I. Elomaa: Aminoterminal propeptide of type I procollagen (PINP) correlates to bone loss and predicts the efficacy of antiresorptive therapy in pre- and post -menopausal non-metastatic breast cancer patients. In: British journal of cancer Volume 78, Number 2, July 1998, pp. 240-245, PMID 9683300 . PMC 2062893 (free full text).
- ↑ D. Pollmann, S. Siepmann, R. Geppert, KD Wernecke, K. Possinger, D. Lüftner: The amino-terminal propeptide (PINP) of type I collagen is a clinically valid indicator of bone turnover and extent of metastatic spread in osseous metastatic breast cancer. In: Anticancer Research Volume 27, Number 4A, 2007 Jul-Aug, pp. 1853-1862, PMID 17649784 .
- ↑ Würmtal Diagnostics: I-carboxy-terminal telopeptide (ICTP) - catabolic bone marker. (PDF; 27 kB) September 1, 2008
- ↑ AM Salem, SF Zohny, MM Abd El-Wahab, R. Hamdy: Predictive value of osteocalcin and beta-CrossLaps in metastatic breast cancer. In: Clinical biochemistry Volume 40, Number 16-17, November 2007, pp 1201-1208, doi : 10.1016 / j.clinbiochem.2007.07.006 . PMID 17889845 .
- ^ TA Gardner, SJ Lee, SD Lee, X. Li, T. Shirakawa, DD Kwon, RY Park, KY Ahn, C. Jung: Differential expression of osteocalcin during the metastatic progression of prostate cancer. In: Oncology reports Volume 21, Number 4, April 2009, pp. 903-908, PMID 19287987 .
- ^ A b Y. Gao, H. Lu, Q. Luo, X. Wu, S. Sheng: Predictive value of osteocalcin in bone metastatic differentiated thyroid carcinoma. In: Clinical biochemistry Volume 43, Number 3, February 2010, pp. 291-295, doi : 10.1016 / j.clinbiochem.2009.08.015 . PMID 19732762 .
- ↑ LY Shih, HN Shih, TH Chen: Bone resorption activity of osteolytic metastatic lung and breast cancers. In: Journal of orthopedic research Volume 22, Number 6, November 2004, pp. 1161–1167, doi : 10.1016 / j.orthres.2004.03.004 . PMID 15475192 .
- ↑ SJ Hotte, EW Winquist, L. Stitt, SM Wilson, AF Chambers: plasma osteopontin: associations with survival and metastasis to bone in men with hormone-refractory prostate carcinoma. In: Cancer Volume 95, Number 3, August 2002, pp. 506-512, doi : 10.1002 / cncr.10709 . PMID 12209742 .
- ↑ A. Ramankulov, M. Lein, G. Kristiansen, SA Loening, K. Jung: Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. In: The Prostate Volume 67, Number 3, February 2007, pp. 330-340, doi : 10.1002 / pros.20540 . PMID 17192877 .
- ↑ A. Macrì, A. Versaci, G. Lupo, G. Trimarchi, C. Tomasello, S. Loddo, G. Sfuncia, R. Caminiti, D. Teti, C. Famulari: Role of osteopontin in breast cancer patients. In: Tumori Volume 95, Number 1, 2009 Jan-Feb, pp. 48-52, PMID 19366056 .
- ↑ K. Mado, Y. Ishii, T. Mazaki, M. Ushio, H. Masuda, T. Takayama: A case of bone metastasis of colon cancer that markedly responded to S-1 / CPT-11 combination chemotherapy and became curable by resection. In: World journal of surgical oncology Volume 4, 2006, p. 3, doi : 10.1186 / 1477-7819-4-3 . PMID 16417646 . PMC 1395313 (free full text). ( Open access )
- ↑ International Atomic Energy Agency: Selection of Labeling Kit. Retrieved March 11, 2011
- ^ RE O'Mara: Skeletal scanning in neoplastic disease. In: Cancer Volume 37, number 1 suppl, January 1976, pp. 480-486, PMID 1247979 .
- ↑ N. Alazraki, D. Resnick: Radionuclide techniques. In: D. Resnick (Ed.): Bone and joint imaging WB Saunders, 1989, ISBN 0-7216-2215-1 , pp. 185-198.
- ↑ N. Otsuka, M. Fukunaga, K. Morita, S. Ono, K. Nagai: Photon-deficient finding in sternum on bone scintigraphy in patients with malignant disease. In: Radiation medicine Volume 8, Number 5, 1990, pp. 168-172, PMID 2075233 .
- ^ RS Hellman, MA Wilson: Discordance of sclerosing skeletal secondaries between sequential scintigraphy and radiographs. In: Clinical Nuclear Medicine Volume 7, Number 3, March 1982, pp. 97-98, PMID 7060310 .
- ↑ FL Datz, GG Patch, JM Arias, KA Morton: Nuclear medicine. A teaching file. Mosby Year Book, 1992, ISBN 0-8016-6365-2 , pp. 28-29.
- ↑ WT Yuh, CK Zachar, TJ Barloon, Y. Sato, WJ Sickels, DR Hawes: Vertebral compression fractures: distinction between benign and malignant causes with MR imaging. In: Radiology Volume 172, Number 1, July 1989, pp. 215-218, PMID 2740506 .
- ↑ A. Baur, A. Stäbler, S. Arbogast, HR Duerr, R. Bartl, M. Reiser: Acute osteoporotic and neoplastic vertebral compression fractures: fluid sign at MR imaging. In: Radiology Volume 225, Number 3, December 2002, pp. 730-735, PMID 12461253 .
- ↑ J. Kollath: Radiological diagnosis of bone metastases. In: H. Böttcher, I. Adamietz (Ed.): Clinic of Skeletal Metastases: Basics, Diagnostics, Therapy. Zuckschwerdt, ISBN 3-88603-587-5 , pp. 8-13.
- ↑ NA Ebraheim, RE Rupp, ER Savolaine, D. Reinke: Use of titanium implants in pedicular screw fixation. In: Journal of spinal disorders Volume 7, Number 6, December 1994, pp. 478-486, PMID 7873844 .
- ↑ Paul Shreve, David W. Townsend: Clinical PET-CT in Radiology Integrated Imaging in Oncology Springer Science + Business Media, LLC 2011 ISBN 978-0-387-48900-1 , Chapter 32: PET-CT of Bone Metastases, by James A. Scott and Edwin L. Palmer
- ^ AD Johnston: Pathology of metastatic tumors in bone. In: Clinical Orthopedics and Related Research Volume 73, 1970, pp. 8-32, PMID 4920992 . (Review).
- ↑ CR Dunstan, D. Felsenberg, MJ Seibel: Therapy insight: the risks and benefits of bisphosphonates for the treatment of tumor-induced bone disease. In: Nature clinical practice. Oncology Volume 4, Number 1, January 2007, pp. 42-55, doi : 10.1038 / ncponc0688 . PMID 17183355 . (Review).
- ^ AC Hindley, EB Hill, MJ Leyland, AE Wiles: A double-blind controlled trial of salmon calcitonin in pain due to malignancy. In: Cancer chemotherapy and pharmacology Volume 9, Number 2, 1982, pp. 71-74, PMID 6756666 .
- ^ D. Lussier, AG Huskey, RK Portenoy: Adjuvant analgesics in cancer pain management. In: The oncologist Volume 9, Number 5, 2004, pp. 571-591, doi : 10.1634 / theoncologist.9-5-571 . PMID 15477643 . (Review).
- ^ A. Roth, K. Kolaric: Analgetic activity of calcitonin in patients with painful osteolytic metastases of breast cancer. Results of a controlled randomized study. In: Oncology Volume 43, Number 5, 1986, pp. 283-287, PMID 3531953 .
- ^ J. Szántó, N. Ady, S. József: Pain killing with calcitonin nasal spray in patients with malignant tumors. In: Oncology Volume 49, Number 3, 1992, pp. 180-182, PMID 1495743 .
- ^ MJ Martinez-Zapata, M. Roqué, P. Alonso-Coello, E. Català: Calcitonin for metastatic bone pain. In: Cochrane database of systematic reviews (Online) Volume 3, 2006, S. CD003223, doi : 10.1002 / 14651858.CD003223.pub2 . PMID 16856000 . (Review).
- ↑ MJ Martinez, M. Roqué, P. Alonso-Coello, E. Català, JL Garcia, M. Ferrandiz: Calcitonin for metastatic bone pain. In: Cochrane database of systematic reviews (Online) Number 3, 2003, S. CD003223, doi : 10.1002 / 14651858.CD003223 . PMID 12917954 . (Review).
- ^ HJ McQuay, SL Collins, D. Carroll, RA Moore: Radiotherapy for the palliation of painful bone metastases. In: Cochrane database of systematic reviews (Online) Number 2, 2000, S. CD001793, doi : 10.1002 / 14651858.CD001793 . PMID 10796822 . (Review).
- ^ P. Koehler: Radiation therapy of bone metastases. ( Memento of the original from March 31, 2010 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 606 kB) In: radiologen-konstanz.de 4, 2005
- ↑ Palliative pain therapy for skeletal metastases. ( Memento of the original from May 30, 2010 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Ulm University Hospital, accessed on February 25, 2011
- ^ EB Silberstein: Teletherapy and radiopharmaceutical therapy of painful bone metastases. In: Seminars in nuclear medicine Volume 35, Number 2, April 2005, pp. 152–158, doi : 10.1053 / j.semnuclmed.2004.11.006 . PMID 15765378 . (Review).
- ^ A b D. A. Loblaw, JS Wu, P. Kirkbride, T. Panzarella, K. Smith, J. Aslanidis, P. Warde: Pain flare in patients with bone metastases after palliative radiotherapy - a nested randomized control trial. In: Supportive care in cancer Volume 15, Number 4, April 2007, pp. 451-455, doi : 10.1007 / s00520-006-0166-y . PMID 17093912 .
- ↑ European Medicines Agency : Xofigo. Radium-223 dichloride (PDF; 75 kB). EMA / 579264/2013, EMEA / H / C / 002653, November 13, 2013.
- ↑ a b J. T. Buijs, CC Kuijpers, G. van der Pluijm: Targeted therapy options for treatment of bone metastases; beyond bisphosphonates. In: Current pharmaceutical design Volume 16, Number 27, 2010, pp. 3015-3027, PMID 20722621 . (Review).
- ↑ CH Van Poznak, S. Temin u. a .: American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. In: Journal of clinical oncology . Volume 29, number 9, March 2011, pp. 1221-1227, doi : 10.1200 / JCO.2010.32.5209 . PMID 21343561 .
- ^ RE Coleman, EV McCloskey: Bisphosphonates in oncology. In: Bone. Volume 49, number 1, July 2011, pp. 71-76, doi : 10.1016 / j.bone.2011.02.003 . PMID 21320652 . (Review).
- ^ EJ Woodward, RE Coleman: Prevention and treatment of bone metastases. In: Current pharmaceutical design Volume 16, Number 27, 2010, pp. 2998-3006, PMID 20722624 . (Review).
- ^ I. Schirmer, H. Peters, PA Reichart and H. Dürkop: bisphosphonates and osteonecroses in the jaw area. In: Oral, Maxillofacial and Facial Surgery Volume 9, Number 4, 2005, pp. 239–245. doi : 10.1007 / s10006-005-0616-6 PMID 15918066
- ↑ L. Raffaelli, L. Scaramuzzo, P. Rossi Iommetti, C. Graci, G. Maccauro, PF Manicone: Jaw osteonecrosis related to bisphosphonate treatment of bone metastasis. In: Journal of biological regulators and homeostatic agents Volume 24, Number 2, 2010, pp. 115-121, PMID 20487624 .
- ↑ PG Fournier, V. Stresing, FH Ebetino, P. Clézardin: How do bisphosphonates inhibit bone metastasis in vivo? In: Neoplasia Volume 12, Number 7, July 2010, pp. 571-578, PMID 20651986 . PMC 2907583 (free full text).
- ^ IJ Diel: Antitumoral effects of bisphosphonates. In: Drugs Volume 59, Number 3, March 2000, pp. 391-399, PMID 10776826 . (Review).
- ↑ H. Almubarak, A. Jones, R. Chaisuparat, M. Zhang, TF Meiller, MA Scheper: Zoledronic acid directly suppresses cell proliferation and induces apoptosis in highly tumorigenic prostate and breast cancers. In: Journal of carcinogenesis Volume 10, 2011, p. 2, doi : 10.4103 / 1477-3163.75723 . PMID 21297922 . PMC 3030761 (free full text).
- ↑ XGEVA ® 120 mg solution for injection (PDF; 123 kB) Technical information XGEVA ® (summary of the product characteristics), as of August 2012
- ↑ Xgeva ® (denosumab) (PDF; 361 kB), New Medicines - Information from the Medicines Commission of the German Medical Association (AkdÄ)
- ↑ Denosumab for bone metastases: Improving the quality of life of patients , JOURNAL ONKOLOGIE, magazine online - issue 07–11
- ^ Oncology: Antibodies slows down bone loss , Dtsch Arztebl 2010; 107 (3): A-98
- ↑ Prolia ® 60 mg solution for injection (PDF; 123 kB) Specialist information Prolia ® (summary of the product characteristics), as of August 2012
- ↑ Prostate Cancer: Hormonal Therapy and Hormone Withdrawal , Cancer Information Service of the German Cancer Research Center (DKFZ), Heidelberg. As of July 31, 2010, last accessed on September 4, 2014
- ↑ P. Schöffski: Alopecia after chemotherapy. In: Compendium of internal oncology. 2006, 33, pp. 2244-2247. doi : 10.1007 / 3-540-31303-6_138
- ↑ Bone metastases in renal cell carcinoma: renal cell carcinoma
- ↑ T. Wirth, P. Griss: The operative therapy of bone metastasis. In: Der Urologe Volume 38, Number 3, 1998, pp. 226-230. doi : 10.1007 / s001310050191
- ↑ General procedure for spinal metastases: http://www.tumororthopaedie.org/uebersicht-3.html
- ↑ Dorsal instrumentation on the spine: http://www.tumororthopaedie.org/stabilisierung-dorsal.html
- ^ Ventral spine stabilization: http://www.tumororthopaedie.org/stabilisierung-ventral.html
- ↑ bone metastases. ( Memento of the original from November 16, 2012 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. Heidelberg University Hospital, accessed on February 28, 2011
- ↑ Vertebro- and kyphoplasty: http://www.tumororthopaedie.org/vertebro--kyphoplastie.html
- ↑ Tumor endoprostheses (megaprostheses): http://www.tumororthopaedie.org/uebersicht-1.html
- ↑ Diaphyseal prostheses: http://www.tumororthopaedie.org/untere-extremitaet.html
- ↑ TO Kurup, MR Callstrom: Ablation of skeletal metastases: current status. In: Journal of vascular and interventional radiology Volume 21, Number 8 Suppl, August 2010, pp. S242 – S250, doi : 10.1016 / j.jvir.2010.05.001 . PMID 20656234 . (Review).
- ↑ MD Lane, HB Le, S. Lee, C. Young, MK Heran, M. Badii, PW Clarkson, PL Munk: Combination radiofrequency ablation and cementoplasty for palliative treatment of painful neoplastic bone metastasis: experience with 53 treated lesions in 36 patients . In: Skeletal radiology Volume 40, Number 1, January 2011, pp. 25-32, doi : 10.1007 / s00256-010-1010-5 . PMID 20686765 .
- ↑ PL Munk, F. Rashid, MK Heran, M. Papirny, DM Liu, D. Malfair, M. Badii, PW Clarkson: Combined cementoplasty and radiofrequency ablation in the treatment of painful neoplastic lesions of bone. In: Journal of vascular and interventional radiology Volume 20, Number 7, July 2009, pp. 903-911, doi : 10.1016 / j.jvir.2009.03.035 . PMID 19481469 .
- ↑ K. Wilhelm: Osteoplasty in combination with radio frequency ablation for the palliative therapy of painful osteolytic bone metastases. In: Deutsche Zeitschrift für Onkologie Volume 41, Number 4, 2009, pp. 183–186. doi : 10.1055 / s-0029-1242535
- ↑ AF Posteraro, DE Dupuy, WW Mayo-Smith: Radio Frequency Ablation of bony metastatic disease. In: Clinical radiology Volume 59, Number 9, September 2004, pp. 803-811, doi : 10.1016 / j.crad.2004.01.015 . PMID 15351245 . (Review).
- ^ G. Belfiore, E. Tedeschi, FM Ronza, MP Belfiore, T. Della Volpe, G. Zeppetella, A. Rotondo: Radiofrequency ablation of bone metastases induces long-lasting palliation in patients with untreatable cancer. (PDF; 351 kB) In: Singapore medical journal Volume 49, Number 7, July 2008, pp. 565-570, PMID 18695866 .
- ↑ MR Callstrom, JW Charboneau: Image-guided palliation of painful metastases using percutaneous ablation. In: Techniques in vascular and interventional radiology Volume 10, Number 2, June 2007, pp. 120-131, doi : 10.1053 / j.tvir.2007.09.003 . PMID 18070690 . (Review).
- ↑ L. Thanos, S. Mylona, P. Galani, D. Tzavoulis, V. Kalioras, S. Tanteles, M. Pomoni: Radiofrequency ablation of osseous metastases for the palliation of pain. In: Skeletal radiology Volume 37, Number 3, March 2008, pp. 189-194, doi : 10.1007 / s00256-007-0404-5 . PMID 18030464 . PMC 2226078 (free full text). (Review).
- ↑ A. Gillams: ablation Tumor: current role in the liver, kidney, lung and bone. In: Cancer imaging Volume 8, 2008, pp. S1-S5, doi : 10.1102 / 1470-7330.2008.9001 (currently not available) . PMID 18852074 . PMC 2582496 (free full text). (Review).
- ↑ MR Callstrom, TD Atwell, JW Charboneau, MA Farrell, MP Goetz, J. Rubin, JA Sloan, PJ Novotny, TJ Welch, TP Maus, GY Wong, KJ Brown: Painful metastases involving bone: percutaneous image-guided cryoablation-prospective trial interim analysis. ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. In: Radiology Volume 241, Number 2, November 2006, pp. 572-580, doi : 10.1148 / radiol.2412051247 . PMID 17057075 .
- ↑ DE Dupuy, D. Liu, D. Hartfeil, L. Hanna, JD Blume, K. Ahrar, R. Lopez, H. Safran, T. DiPetrillo: Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. In: Cancer Volume 116, Number 4, February 2010, pp. 989-997, doi : 10.1002 / cncr.24837 . PMID 20041484 . PMC 2819592 (free full text).
- ↑ M. Kashima, K. Yamakado, H. Takaki, T. Kaminou, N. Tanigawa, A. Nakatsuka, K. Takeda: Radiofrequency ablation for the treatment of bone metastases from hepatocellular carcinoma. In: American Journal of Roentgenology Volume 194, Number 2, February 2010, pp. 536-541, doi : 10.2214 / AJR.09.2975 . PMID 20093621 .
- ↑ G. Carrafiello, D. Laganà, A. Ianniello, P. Nicotera, F. Fontana, M. Dizonno, S. Cuffari, C. Fugazzola: Radiofrequency thermal ablation for pain control in patients with single painful bone metastasis from hepatocellular carcinoma. In: European journal of radiology Volume 71, Number 2, August 2009, pp. 363-368, doi : 10.1016 / j.ejrad.2008.04.019 . PMID 18514456 .
- ↑ G. Orgera, L. Monfardini et al. a .: High-intensity focused ultrasound (HIFU) in patients with solid malignancies: evaluation of feasibility, local tumor response and clinical results. In: La Radiologia medica. Volume 116, Number 5, August 2011, pp. 734-748, doi : 10.1007 / s11547-011-0634-4 . PMID 21293939 .
- ^ A. Gangi, B. Kastler, A. Klinkert, JL Dietemann: Injection of alcohol into bone metastases under CT guidance. In: Journal of computer assisted tomography Volume 18, Number 6, 1994 Nov-Dec, pp. 932-935, PMID 7962803 .
- ^ JC Clark, CR Dass, PF Choong: Current and future treatments of bone metastases. In: Expert opinion on emerging drugs Volume 13, Number 4, December 2008, pp. 609–627, doi : 10.1517 / 14728210802584217 . PMID 19046130 . (Review).
- ^ EM Lewiecki: Odanacatib, a cathepsin K inhibitor for the treatment of osteoporosis and other skeletal disorders associated with excessive bone remodeling. In: IDrugs Volume 12, Number 12, December 2009, pp. 799-809, PMID 19943223 . (Review).
- ↑ clinicaltrials.gov: Efficacy of RAD001 in Breast Cancer Patients With Bone Metastases (RADAR). dated July 21, 2010, accessed March 7, 2011
- Jump up ↑ A. Russo, G. Bronte, S. Rizzo, D. Fanale, F. Di Gaudio, N. Gebbia, V. Bazan: Anti-endothelin drugs in solid tumors. In: Expert opinion on emerging drugs Volume 15, Number 1, March 2010, pp. 27-40, doi : 10.1517 / 14728210903571667 . PMID 20102289 . (Review).
- ↑ National Cancer Institute: Anti-M-CSF monoclonal antibody MCS110. Retrieved March 7, 2011
- ^ T. Onishi, N. Hayashi, RL Theriault, GN Hortobagyi, NT Ueno: Future directions of bone-targeted therapy for metastatic breast cancer. In: Nature reviews. Clinical oncology Volume 7, Number 11, November 2010, pp. 641-651, doi : 10.1038 / nrclinonc.2010.134 . PMID 20808302 .
- ↑ D. Santini, S. Galluzzo, A. Zoccoli, F. Pantano, ME Fratto, B. Vincenzi, L. Lombardi, C. Gucciardino, N. Silvestris, E. Riva, S. Rizzo, A. Russo, E. Maiello, G. Colucci, G. Tonini: New molecular targets in bone metastases. In: Cancer treatment reviews Volume 36 Suppl 3, November 2010, pp. S6-S10, doi : 10.1016 / S0305-7372 (10) 70013-X . PMID 21129612 . (Review).
- ↑ a b P. Feyer, W. Haase, u. a .: Guidelines: Palliative radiation therapy (general). Retrieved March 1, 2011
- ^ RE Coleman, P. Smith, RD Rubens: Clinical course and prognostic factors following bone recurrence from breast cancer. In: British Journal of Cancer Volume 77, Number 2, 1998, pp. 336-340, PMID 9461007 . PMC 2151240 (free full text).
- ↑ K. Fan, CF Peng: Predicting the probability of bone metastasis through histological grading of prostate carcinoma: a retrospective correlative analysis of 81 autopsy cases with antemortem transurethral resection specimen. In: The Journal of urology Volume 130, Number 4, October 1983, pp. 708-711, PMID 6350620 .
- ↑ PG Koenders, LV Beex, PW Kloppenborg, AG Smals, TJ Benraad: Human breast cancer: survival from first metastasis. Breast Cancer Study Group. In: Breast Cancer Research and Treatment Volume 21, Number 3, 1992, pp. 173-180, PMID 1515652 .
- ↑ EF Solomayer, IJ Diel, GC Meyberg, C. Gollan, G. Bastert: Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. In: Breast Cancer Research and Treatment Volume 59, Number 3, February 2000, pp. 271-278, PMID 10832597 .
- ^ RJ Cook, P. Major: Multistate analysis of skeletal events in patients with bone metastases. In: Clinical Cancer Research Volume 12, Number 20, October 2006, pp. 6264s-6269s, doi : 10.1158 / 1078-0432.CCR-06-0654 . PMID 17062711 . (Review).
- ↑ HC Bauer, R. Wedin: Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. In: Acta orthopaedica Scandinavica Volume 66, Number 2, April 1995, pp. 143-146, PMID 7740944 .
- ↑ RK Chhem, DR Brothwell: Paleoradiology: Imaging Mummies and Fossils. Springer, 2007, ISBN 3-540-48832-4 , p. 101. Restricted preview in the Google book search
- ^ Paleontologists Teach Medical Students About Fossil Tumors. ( Memento of the original from March 16, 2011 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. In: Science Daily June 1, 2006.
- ↑ a b c M. Kessler: Small animal oncology - diagnosis and therapy of tumor diseases in dogs and cats. Georg Thieme Verlag, 2005, ISBN 3-8304-4103-7 limited preview in the Google book search
- ↑ RW Nelson, CG Couto: Internal medicine of small animals. Urban & Fischer, 2006, ISBN 3-437-57040-4 , p. 1229. limited preview in Google book search