Atrasentan
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Atrasentan | |||||||||||||||||||||
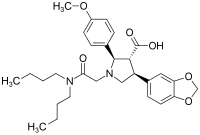
| other names |
(2 R , 3 R , 4 S ) -1 - [(Dibutylcarbamoyl) methyl] -2- ( p -methoxyphenyl) -4- [3,4- (methylenedioxy) phenyl] -3-pyrrolidinecarboxylic acid ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 29 H 38 N 2 O 6 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 510.62 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Atrasentan (ABT-627) is an experimental drug being developed to treat prostate cancer and albuminuria associated with diabetic nephropathy .
The cytostatic effect of atrasentan comes about through its antagonistic binding to the endothelin -A receptor. Thereby it reduces cell proliferation and metastasis of tumor cells . Atrasentan is about to be launched in the United States as Xinlay ® (manufacturer: Abbott ).
literature
Articles on analytics
- Bryan et al .: Determination of Atrasentan by high performance liquid chromatography with fluorescence detection in human plasma ., In: Biomed Chromatogr . , 15/8, 2001, pp. 525-533.
- Morley et al .: Determination of the endothelin receptor antagonist ABT-627 and related substances by high performance liquid chromatography , In: J Pharm Biomed Anal . , 7, 1999, pp. 777-784.
Articles on synthesis
- Boyd et al .: Discovery of a Series of Pyrrolidine-based Endothelin Receptor Antagonists with Enhanced ET A Receptor Selectivity . In: Bio-Med-Chem. 7, 1999, pp. 991-1002.
Articles on pharmacology
- MA Carducci, A. Jimeno: Targeting bone metastasis in prostate cancer with endothelin receptor antagonists. In: Clin Cancer Res . 2006 Oct 15; 12 (20 Pt 2): 6296s-6300s, PMID 1706271 .
- Hammad et al .: Role of endothelin ET A Receptor antagonism in the post-transplant renal response to angiotensin II in rats ., In: Exp. Physiol. , 83/3, 2001, 365-372.
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of (2R, 3R, 4S) -4- (2H-1,3-benzodioxol-5-yl) -1 - [(dibutylcarbamoyl) methyl] -2- (4-methoxyphenyl) is shown, which is derived from a self-classification by the distributor ) pyrrolidine-3-carboxylic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 17, 2019.
- ↑ Clinical Study (Phase II): Evaluate the Efficacy and Safety of Once Daily Administration of Atrasentan Tablets (Low and High) Compared to Placebo in Reducing Residual Albuminuria in Type 2 Diabetic Patients With Nephropathy Who Are Treated With the Maximum Tolerated Labeled Dose of a Renin Angiotensin System (RAS) Inhibitor (RADAR) at Clinicaltrials.gov of the NIH
- ↑ Clinical Study (Phase II): A Study of Atrasentan on Reducing Albuminuria in Type 2 Diabetic Nephropathy Treated With Renin-Angiotensin System Inhibitors at Clinicaltrials.gov of the NIH
- ↑ ABDA database (as of December 4, 2009).