Osteoclast
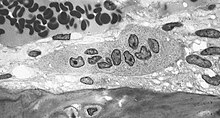
Osteoclasts (from the Greek ὀστέον (ostéon) bone and κλᾰστός (klastós) broken), ( singular osteoclast ) are multinucleated cells that are formed by the fusion of mononuclear progenitor cells from the bone marrow . They belong to the mononuclear phagocytic system (MPS). Its main task is the resorption of bone tissue .
Osteoclasts develop from hematopoietic stem cells in the bone marrow . Some of them show properties of circulating monocytes and tissue macrophages .
regulation
The following factors can stimulate the formation of osteoclasts and thus have a bone resorptive effect:
- Dexamethasone
- 1,25- (OH) 2 VitD 3
- Parathyroid hormone
- Parathyroid hormone-related protein (PTHrP)
- Peroxisome Proliferator Activated Receptor-γ (PPARγ)
- Prostaglandin E2
- Triiodothyronine (T3)
- different cytokines
The following factors can inhibit the function of osteoclasts and thus have an antiresorptive effect:
These factors lead to an activation of the PU.1 transcription factor, which increases the conversion of macrophages into osteoclasts. Osteoclasts are also activated via the RANK ligand (RANKL) . Osteoprotegerin (OPG) is a protein that inhibits osteoclast differentiation by binding the RANK ligands. For their part, these ligands can no longer bind to the RANK of the macrophages and can therefore no longer stimulate them to differentiate into osteoclasts. In this way, osteoprotegerin prevents the breakdown of bones.
The osteoclast activity is hormonally controlled by parathyroid hormone (activation, calcium release) and calcitonin (inactivation, calcium storage), so the bone is used as an intermediate store of calcium to regulate calcium homeostasis . It should be noted, however, that the osteoclast itself does not have any receptors for the parathyroid hormone, but is activated secondarily via the osteoblasts (RANKL), which have this receptor. However, the osteoclast has receptors for calcitonin, which can directly inhibit its activity.
The resorption of bone is usually closely linked to a subsequent new synthesis by osteoblasts . This process, known as bone tissue remodeling, is used to adapt to loads and to prevent material fatigue.
histology
Osteoclasts are large cells with a diameter of 50 to 100 µm that can contain up to 10 cell nuclei . They are found on the bone surface in the resorption lacunae (Howship lacunae). Just like the closely related macrophages , they are able to move amoeboid .
The apical pole of an osteoclast faces the bone. Different areas can be distinguished here:
- In the center there is a bright, streaky and vesicle-rich zone in which the cell membrane is strongly folded in the form of a brush border . It is known as the ruffled border and is the actual site of bone resorption . Under the light microscope, it appears light-striped.
- A light microscope shows an intense color in the periphery. There, the osteoclast adheres to the bone substance with its adhesion apparatus ( integrins ). The adhesion apparatus mostly consists of so-called podosomes . There is very close contact between the osteoclast and the bone, with a distance of only 0.3–0.5 nm. This area is therefore also called the sealing zone .
- The cytoplasm that surrounds the sealing zone is called the clear zone , as it has almost no cell organelles . Instead, there is a large number of contractile proteins here .
physiology
In the space between the osteoclast and the bone substance there is a significantly reduced pH value (approx. 4.5), which is maintained by active proton transport and which serves to break down the mineralized matrix components. In addition, osteoclasts release proteolytic enzymes that dissolve the collagenous bone matrix . The collagen fragments released are phagocytosed . This creates the Howship lacunae, which can also be described as the "feeding track" of the osteoclasts. Their capacity is considerable: one osteoclast can break down the same amount of bone that 100 osteoblasts build up in this time.
Diseases
Decreased osteoclast activity
- In the genetically determined group of diseases of osteopetrosis and its sub-form of pycnodysostosis , osteoclast activity is greatly reduced.
- PLOSL (polycystic lipomembranous osteodysplasia with sclerotic leukoencephalopathy , Nasu-Hakola disease )
Increased osteoclast activity
An increased osteoclast activity is found in the following diseases
- osteoporosis
- Hyperparathyroidism
- Osteodystrophia deformans (Paget's disease)
- Aseptic bone necrosis
- Rheumatoid arthritis
- Osteogenesis imperfecta
- Breast cancer
- Giant cell tumor
- Gorham-Stout Syndrome
See also
literature
- T. Bellido et al: Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. In: J Clin. Invest. 95, 1995, pp. 2886-2895.
- G. Girasole, RL Jilka, G. Passeri, S. Boswell, G. Boder, DC Williams, SC Manolanas: 17-Estradiol inhibits interleukin-6 production by bone marrow derived stromal cells and osteoblasts in vitro: A potential mechanism for the anti -osteoporotic effect of estrogens. In: J. Clin. Invest. 89, 1992, pp. 883-891.
- G. Hattersley, JA Kirby, TJ Chambers: Identification of osteoclast precursors in multilineage hematopoietic colonies. In: Endocrinology. 128, 1991, pp. 259-262.
- RL Jilka et al .: Increased osteoclast development after estrogen loss: Mediation by interleukin-6. In: Science . 257, 1992, pp. 88-91.
- N. Kurihara, C. Chenu, M. Miller, C. Civin, GD Roodman: Identification of committed mononuclear precursors for osteoclast-like cells in long term human marrow cultures. In: Endocrinology. 126, 1990, pp. 2733-2741.
- K. Matsuzaki et al: Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. In: Biochem Biophys Res Commun . 246, 1998, pp. 199-204.
- NK Shevde, AC Bendixen, KM Dienger, JW Pike: Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. In: Proc Natl Acad Sci USA . 97, 2000, pp. 7829-7834.
- MM Tondravi, SR McKercher, K. Anderson, JM Erdmann, M. Quiroz, R. Maki, SL Teitelbaum: Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. In: Nature . 386, 1997, pp. 81-84.
- K. Tsukii et al: Osteoclast differentiation factor mediates an essential signal for bone resorption induced by 1 alpha, 25-dihydroxyvitamin D3, prostaglandin E2, or parathyroid hormone in the microenvironment of bone. In: Biochem Biophys Res Commun. 246, 1998, pp. 337-341.